
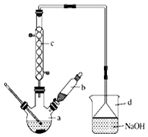
 äå±½ŹĒŅ»Öֻƹ¤ŌĮĻ£¬ŹµŃéŹŅŗĻ³ÉäåĢåµÄ×°ÖĆŹ¾ŅāĶ¼¼°ÓŠ¹ŲŹż¾ŻČē±ķ£ŗ
äå±½ŹĒŅ»Öֻƹ¤ŌĮĻ£¬ŹµŃéŹŅŗĻ³ÉäåĢåµÄ×°ÖĆŹ¾ŅāĶ¼¼°ÓŠ¹ŲŹż¾ŻČē±ķ£ŗ| ±½ | äå | äå±½ | |
| ĆܶČ/g•cm-3 | 0.88 | 3.10 | 1.50 |
| ·Šµć/”ę | 80 | 59 | 156 |
| Ė®ÖŠČܽā¶Č | Ī¢ČÜ | Ī¢ČÜ | Ī¢ČÜ |
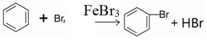 £»×°ÖĆdµÄ×÷ÓĆĪüŹÕHBrŗĶäåÕōĘų£»·ĄÖ¹µ¹Īü£®
£»×°ÖĆdµÄ×÷ÓĆĪüŹÕHBrŗĶäåÕōĘų£»·ĄÖ¹µ¹Īü£®·ÖĪö £Ø1£©ŹµŃéŹŅŗĻ³Éäå±½ŹĒ±½ÓėŅŗäåŌŚĢś·Ū×÷“߻ƼĮµÄĢõ¼žĻĀ·¢ÉśČ”“ś·“Ӧɜ³Éäå±½ŗĶäå»ÆĒā£»HBrÓėŅŗäåŅ×»Ó·¢£¬¶ų±½µÄĀ±“ś·“Ó¦ŹĒ·ÅČČµÄ£¬Ī²ĘųÖŠÓŠHBr¼°»Ó·¢³öµÄBr2£¬ÓĆĒāŃõ»ÆÄĘČÜŅŗĪüŹÕ£¬·ĄÖ¹ĪŪČ¾“óĘų£»
£Ø2£©¢Ł±½ÓėŅŗäåŌŚ“߻ƼĮµÄ×÷ÓĆĻĀ·¢Éś·“Ó¦µÄĶ¬Ź±»¹ÄÜ·ÅČČ£¬¶ų·“Ó¦Īļ±½ŗĶŅŗä嶼Ņ×»Ó·¢£¬ĪŖ¼õÉŁ·“Ó¦Īļ»Ó·¢²¢ĢįøßŌĮĻµÄĄūÓĆĀŹ£¬½«±½ŗĶŅŗäåĄäÄż»ŲĮ÷£»
¢ŚĪŖ¼õÉŁ·“Ó¦Īļ»Ó·¢²¢ĢįøßŌĮĻµÄĄūÓĆĀŹæÉŅŌĶعżĪĀ¶ČµÄæŲÖĘĄ“ŹµĻÖ£¬Ö»ŅŖĪĀ¶ČµĶÓŚ¶žÕߵķŠµć¾ĶæÉŅŌĮĖ£»
£Ø3£©äå±½ÖŠŗ¬ÓŠä壬¼ÓNaOHČÜŅŗ£¬°ŃĪ“·“Ó¦µÄBr2·“Ó¦Ļ“µ½Ė®ÖŠ£»
£Ø4£©ÓÉ·ÖĄė²Ł×÷æÉÖŖ£¬·ÖĄė³öµÄ“Öäå±½ÖŠŗ¬ÓŠĪ“·“Ó¦µÄ±½£¬·ÖĄė»„ČܵÄŅŗĢ壬øł¾Ż·Šµć²»Ķ¬£¬ĄūÓĆÕōĮóµÄ·½·Ø½ųŠŠ·ÖĄė£»
£Ø5£©øł¾ŻÖĘČ”äå±½Ėł¼ÓµÄŅŗĢåµÄĢå»żŅŌ¼°ČÜŅŗµÄĢå»żŅ»°ć²»³¬ČŻĘ÷µÄ$\frac{2}{3}$£¬²»ÉŁÓŚ$\frac{1}{3}$Ą“½ā“š£®
½ā“š ½ā£ŗ£Ø1£©ŹµŃéŹŅŗĻ³Éäå±½ŹĒ±½ÓėŅŗäåŌŚĢś·Ū×÷“߻ƼĮµÄĢõ¼žĻĀ·¢ÉśČ”“ś·“Ӧɜ³Éäå±½ŗĶäå»ÆĒā£¬·“Ó¦·½³ĢŹ½ĪŖ2Fe+3Br2ØT2FeBr3£¬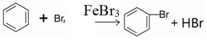 £»HBrÓėŅŗäåŅ×»Ó·¢£¬¶ų±½µÄĀ±“ś·“Ó¦ŹĒ·ÅČČµÄ£¬Ī²ĘųÖŠÓŠHBr¼°»Ó·¢³öµÄBr2£¬ÓĆĒāŃõ»ÆÄĘČÜŅŗĪüŹÕ£¬·ĄÖ¹ĪŪČ¾“óĘų£¬µ¹æŪĀ©¶·»¹ÄÜ·ĄÖ¹µ¹Īü£»
£»HBrÓėŅŗäåŅ×»Ó·¢£¬¶ų±½µÄĀ±“ś·“Ó¦ŹĒ·ÅČČµÄ£¬Ī²ĘųÖŠÓŠHBr¼°»Ó·¢³öµÄBr2£¬ÓĆĒāŃõ»ÆÄĘČÜŅŗĪüŹÕ£¬·ĄÖ¹ĪŪČ¾“óĘų£¬µ¹æŪĀ©¶·»¹ÄÜ·ĄÖ¹µ¹Īü£»
¹Ź“š°ø£ŗ£¬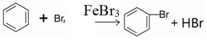 £»ĪüŹÕHBrŗĶäåÕōĘų£»·ĄÖ¹µ¹Īü£»
£»ĪüŹÕHBrŗĶäåÕōĘų£»·ĄÖ¹µ¹Īü£»
£Ø2£©¢Ł±½ÓėŅŗäåŌŚ“߻ƼĮµÄ×÷ÓĆĻĀ·¢Éś·“Ó¦µÄĶ¬Ź±»¹ÄÜ·ÅČČ£¬¶ų·“Ó¦Īļ±½ŗĶŅŗä嶼Ņ×»Ó·¢£¬ĪŖ¼õÉŁ·“Ó¦Īļ»Ó·¢²¢ĢįøßŌĮĻµÄĄūÓĆĀŹ£¬½«±½ŗĶŅŗäåĄäÄż»ŲĮ÷£»
¹Ź“š°øĪŖ£ŗC6H6”¢Br2£»
¢Śøł¾Ż±ķÖŠ±½ŗĶŅŗäåµÄ·Šµć£¬Ń”ŌńæŲÖĘĪĀ¶Č£¬Č·±£ĪĀ¶ČŌŚ¶žÖÖ·“Ó¦ĪļµÄ·ŠµćÖ®ĻĀ£¬ŌņӦєŌńĪĀ¶ČĪŖµĶÓŚ59”ę£»
¹Ź“š°øĪŖ£ŗC£»
£Ø3£©äå±½ÖŠŗ¬ÓŠä壬¼ÓNaOHČÜŅŗ£¬°ŃĪ“·“Ó¦µÄBr2±ä³ÉNaBrŗĶNaBrOĻ“µ½Ė®ÖŠ£¬·½³ĢĪŖ£ŗBr2+2NaOH=NaBr+NaBrO+H2O£»
¹Ź“š°øĪŖ£ŗBr2+2NaOH=NaBr+NaBrO+H2O£»
£Ø4£©·“Ó¦ŗóµĆµ½µÄäå±½ÖŠČÜÓŠÉŁĮæĪ“·“Ó¦µÄ±½£¬±½ŗĶäå±½»„ČÜ£¬µ«±½µÄ·ŠµćµĶ£¬ĖłŅŌ²ÉÓĆÕōĮóµÄ·½·Ø½ųŠŠ·ÖĄė£¬äå±½ĮōŌŚÄøŅŗÖŠ£»
¹Ź“š°øĪŖ£ŗ±½£»C£»
£Ø5£©²Ł×÷¹ż³ĢÖŠ£¬ĻČŌŚaÖŠ¼ÓČė15mLĪŽĖ®±½£¬ŌŚbÖŠŠ”ŠÄ¼ÓČė4.0mLŅŗĢ¬ä壬×īŗóĻņaÖŠ¼ÓČė10mLĖ®£¬¹²Ō¼30mL£¬ĖłŅŌaµÄČŻ»ż×īŹŹŗĻµÄŹĒ50mL£»
¹Ź“š°øĪŖ£ŗB£®
µćĘĄ ±¾Ģāæ¼²éĪļÖŹÖʱøŹµŃé·½°øµÄÉč¼Ę£¬ĪŖøßĘµæ¼µć£¬ĢāÄæÄѶČÖŠµČ£¬Ć÷Č·ŹµŃéŌĄķŹĒ½ā±¾Ģā¹Ų¼ü£¬ÖŖµĄŹµŃé²Ł×÷²½Öč¼°»ł±¾²Ł×÷·½·Ø£¬ŹŌĢāÅąŃųĮĖѧɜµÄ·ÖĪöÄÜĮ¦¼°»ÆѧŹµŃéÄÜĮ¦£®


 ÅąÓÅŗĆ¾ķµ„ŌŖ¼ÓĘŚÄ©¾ķĻµĮŠ“š°ø
ÅąÓÅŗĆ¾ķµ„ŌŖ¼ÓĘŚÄ©¾ķĻµĮŠ“š°ø Ņ»ĻßĆūŹ¦ČØĶž×÷Ņµ±¾ĻµĮŠ“š°ø
Ņ»ĻßĆūŹ¦ČØĶž×÷Ņµ±¾ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
| ŹµŃ鱹ŗÅ | c£ØHA£©/mol•L-1 | c£ØNaOH£©/mol•L-1 | »ģŗĻČÜŅŗµÄpH |
| I | 0.2 | 0.2 | pH=a |
| II | c1 | 0.2 | pH=7 |
| III | 0.2 | 0.1 | pH£¾7 |
| IV | 0.1 | 0.1 | pH=9 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¾Ū±ūĻ©µÄ½į¹¹¼ņŹ½£ŗ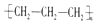 | B£® | ±ūĶé·Ö×ӵıȥżÄ£ŠĶ£ŗ | ||
| C£® | ŅŅČ²µÄµē×ÓŹ½£ŗ | D£® | 2-ŅŅ»ł-1£¬3-¶”¶žĻ©·Ö×ӵļüĻߏ½£ŗ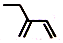 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| ŹµŃéÄŚČŻ | ŹµŃéÄæµÄ | |
| A | Ļņ1mL 1%µÄNaOHČÜŅŗÖŠ¼ÓČė2mL 2%µÄCuSO4ČÜŅŗ£¬Õńµ“ŗóŌŁ¼ÓČė0.5mLÓŠ»śĪļX£¬¼ÓČČÖó·ŠŗóĪ“³öĻÖשŗģÉ«³Įµķ | ŃéÖ¤X½į¹¹ÖŠ²»ŗ¬ÓŠČ©»ł |
| B | ŌŚ»ģÓŠÉŁĮæ±½·ÓµÄ±½ÖŠµĪ¼Ó×ćĮæäåĖ®£¬³ä·ÖÕńµ“ŗó¹żĀĖ | ³żČ„±½ÖŠÉŁĮæµÄ±½·Ó |
| C | äåŅŅĶéÓėNaOHČÜŅŗ¼ÓČČÖĮ²»ŌŁ·Ö²ć£¬ĄäČ“ŗó¼ÓĻ”ĻõĖįÖĮĖįŠŌ£¬ŌŁµĪ¼ÓAgNO3ČÜŅŗ | ŃéÖ¤Ā±ĖŲŌ×ÓĪŖäåŌ×Ó |
| D | ½«ŅŅ“¼ÓėÅØĮņĖį¹²ČČÖʵƵÄĘųĢåĶØČėĖįŠŌKMnO4ČÜŅŗÖŠ | ¼ģŃéĘųĢåÖŠŗ¬ÓŠŅŅĻ© |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÉżøßĢåĻµµÄĪĀ¶Č»ņŌö¼ÓijŅ»×é·ÖµÄÅØ¶Č¾łÄÜŌö¼Ó·“Ó¦ĢåĻµÖŠ»ī»Æ·Ö×ÓĖłÕ¼°Ł·ÖŹż | |
| B£® | H2+Cl2$\frac{\underline{\;µćČ¼\;}}{\;}$2HCl·“Ó¦ÖŠ»ÆѧÄÜÖ»×Ŗ±äĪŖČČÄÜ | |
| C£® | Ćę·ŪÉś²ś¹¤³§ŅŖĒóŃĻ½ūŃĢ»šŹĒŅņĪŖĆę·ŪÓŠ»śĪļæÅĮ£¼«Š”£¬×ܱķĆ껿¾Ž“óČŻŅ×±»ŅżČ¼±¬ÕØ | |
| D£® | øßĆĢĖį¼ŲŹÜČČ·Ö½āŹĒŅ»øöģŲ¼õŠ”µÄ¹ż³Ģ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
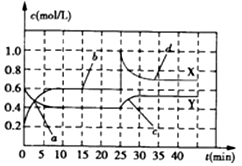
| A£® | Ē°10minÄŚÓĆNO2±ķŹ¾µÄ»Æѧ·“Ó¦ĖŁĀŹv£ØNO2£©=0.02mol/£ØL•min£© | |
| B£® | ·“Ó¦½ųŠŠÖĮ25minŹ±£¬ĒśĻß·¢Éś±ä»ÆµÄŌŅņæÉŅŌŹĒĻņČŻĘ÷ÖŠĢķ¼ÓNO2£Øg£© | |
| C£® | ČōŅŖ“ļµ½Óė×īŗóĻąĶ¬µÄ»ÆŃ§Ę½ŗāדĢ¬£¬ŌŚ25minŹ±»¹æÉŅŌ²ÉČ”µÄ“ėŹ©ŹĒĢķ¼ÓN2O4£Øg£© | |
| D£® | a”¢b”¢c”¢dĖÄøöµćÖŠ£¬±ķŹ¾»Æѧ·“Ó¦“¦ÓŚĘ½ŗāדĢ¬µÄµć³ÉŹĒbŗĶd |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® |  Ķ¼æɱķŹ¾¶ŌĘ½ŗāN2£Øg£©+3H2£Øg£©?2NH3£Øg£©¼ÓŃ¹”¢Ķ¬Ź±ŅĘ³ż²æ·ÖNH3Ź±µÄĖŁĀŹ±ä»Æ | |
| B£® |  Ķ¼ÖŠa”¢bĒśĻßÖ»æɱķŹ¾·“Ó¦H2£Øg£©Ź®I2£Øg£©?2HI£Øg£©ŌŚÓŠ“߻ƼĮŗĶĪŽ“߻ƼĮ“ęŌŚĻĀ½ØĮ¢Ę½ŗāµÄ¹ż³Ģ | |
| C£® | 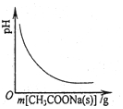 Ķ¼±ķŹ¾ĻņCH3COOHČÜŅŗÖŠÖš½„¼ÓČėCH3COONa¹ĢĢåŗó£¬ČÜŅŗpHµÄ±ä»Æ | |
| D£® | 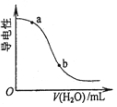 Ķ¼±ķŹ¾Ļņ“×ĖįČÜŅŗÖŠ¼ÓĖ®Ź±Ęäµ¼µēŠŌ±ä»Æ£¬ŌņCH3COOHČÜŅŗµÄpH£ŗa£¼b |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com