
��1���ƶ�A��B��C��D��E�Ļ�ѧʽ��
A��________��B��________��C��________��D��________��E��________��
��2��д���й����ӷ���ʽ��
B�����ᷴӦ��________________________________��
C�����ᷴӦ��________________________________��
D�����ᷴӦ��________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�� | ��ؽṹ������ |
| A | �䵥�ʵ�һ�־��岻�ǽ������壬���ǵ�������壬�����ۡ������������ԣ�������ԭ�Ӻ˷�Ӧ�ѵ���������������������͵缫���ϵȣ� |
| B | �䵥�ʼ�����ǿ����Һ��Ӧ��������ǿ����Һ��Ӧ����������ڵ������ڵ��������а뾶��С�� |
| C | �ǵ����ʵ����Ԫ��֮һ��ԭ�Ӻ��������ֲ�ͬ�����ĵ��ӣ���δ�ɶԵ�������࣮ |
| D | ԭ�Ӻ������������Ų�ʽΪnsnnp2n+1 |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
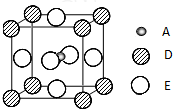 ��2010?̩����ģ����֪��A��B��C��D��EΪ���ڱ�1��36���е�Ԫ�أ����ǵ�ԭ������������A�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�C�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Aԭ����ͬ��C2-����D2+���Ӿ�����ͬ�ġ��ȶ��ĵ��Ӳ�ṹ��E�Ļ�̬ԭ�ӵ���Χ�����Ų�ʽΪ3d84s2��
��2010?̩����ģ����֪��A��B��C��D��EΪ���ڱ�1��36���е�Ԫ�أ����ǵ�ԭ������������A�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�C�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Aԭ����ͬ��C2-����D2+���Ӿ�����ͬ�ġ��ȶ��ĵ��Ӳ�ṹ��E�Ļ�̬ԭ�ӵ���Χ�����Ų�ʽΪ3d84s2���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�� | A | B | C | D | |
| ���ʻ�ṹ��Ϣ | �����µ��ʳ���̬��ԭ��������������D��ͬ�� | D3B���������������ӵĵ��Ӳ�ṹ��ͬ | A��C���γ����ֳ����Ļ�������ң��Ҿ��������� | ��������������ɫ���壬������ǿ���ڿ�����ȼ�����ɵ���ɫ���� | E��һ����̬���������������ڿ������ܶ�Ϊ3.0���ܽ���ˮ�ɵ�ֻ����һ���ʵ���������Һ������Һ�ڹ��������·������Ի���ǿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
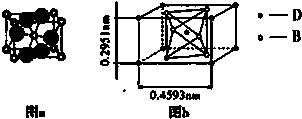
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com