
��������(Na2FeO4)��һ����������������ҵ���Ʊ������������������ַ�����
a��2Fe(OH)3��3NaClO��4NaOH===2Na2FeO4��3NaCl��5H2O��
b��2FeSO4��6Na2O2===2Na2FeO4��2Na2O��2Na2SO4��O2����
c��Fe2O3��3Na2O2===2Na2FeO4��Na2O��
d��Fe(NO3)3��NaOH��Cl2�D��Na2FeO4��NaNO3��NaCl��H2O��
��ش��������⣺
(1)�����ж���ȷ����________(�����)��
A������a��b��c������ˮ��Һ�н���
B������a��b��֪NaClO��Na2O2�������Ծ�ǿ��Na2FeO4��
C��FeSO4ֻ�л�ԭ�ԣ�û��������
D������KSCN��Һ����b�IJ������Ƿ���FeSO4
(2)���ڷ���c����˵����ȷ����________(�����)��
A��Na2O2�������������ǻ�ԭ��
B����ԭ����ֻ��Na2O
C��3 mol Na2O2������Ӧ����6 mol����ת��
D����Na2FeO4��FeΪ��4�ۣ�����ǿ�����ԣ�������ɱ��
(3)���ڷ���d����ش��������⣺
���������뻹ԭ�������ʵ���֮��Ϊ________��
��д��Na2FeO4��H2O��Ӧ�����ӷ���ʽ��____________________________��
�����Ʋ�Na2FeO4����������ɱ���⣬��һ����;��____________________��
������(1)ѡ��A������b��c�ж���Na2O2���뷴Ӧ������ˮ��Һ�н��У�Na2O2��H2O��Ӧ���Ӷ����õ�Na2FeO4��ѡ��B����Ӧ��NaClO��Na2O2����õ��ӣ�Ϊ��������Na2FeO4Ϊ�����������NaClO��Na2O2�������Ծ�ǿ��Na2FeO4�������ԡ�ѡ��C��FeSO4�е�FeԪ��Ϊ��2�ۣ��ڷ�Ӧ�п�ʧȥ���ӣ�Ҳ�ɵõ����ӣ�����FeSO4���������ԣ�Ҳ�л�ԭ�ԡ�ѡ��D��FeSO4������KSCN��Һ��Ӧ����ɫ��Һ��
(2)ѡ��A�����ݷ�Ӧ��Fe2O3��3Na2O2===2Na2FeO4��Na2O��Na2O2�ڷ�Ӧ��ֻ��õ��ӣ�ֻ����������ѡ��B��Na2O2��Ӧ�IJ������Na2FeO4��Na2O�����߶��ǻ�ԭ���ѡ��C,3 mol Na2O2������Ӧ����6 mol����ת�ƣ���ȷ��ѡ��D��Na2FeO4��FeԪ�صĻ��ϼ�Ϊ��6�ۡ�
(3)��������ΪCl2���仯ѧ������Ϊ3����ԭ��ΪFe(NO3)3���仯ѧ������Ϊ2�����ߵ����ʵ���֮��Ϊ3��2���ڸ�����Ŀ��Ϣ����Ӧ��ΪNa2FeO4��H2O��������ΪFe(OH)3��O2��NaOH�����У�Na2FeO4��H2O�D��Fe(OH)3(����)��NaOH��O2������ƽ�ķ�Ӧ��ѧ����ʽΪ4Na2FeO4��10H2O===4Fe(OH)3(����)��8NaOH��3O2���������ӷ�Ӧ����ʽΪ4FeO ��10H2O===4Fe(OH)3(����)��8OH����3O2����Na2FeO4����ǿ�����ԣ������������ɵ�Fe(OH)3������нϴ�ı������������ˮ�е��������ˮ�����á�
��10H2O===4Fe(OH)3(����)��8OH����3O2����Na2FeO4����ǿ�����ԣ������������ɵ�Fe(OH)3������нϴ�ı������������ˮ�е��������ˮ�����á�
�𰸡�(1)B��(2)C
(3)��3��2����4FeO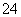 ��10H2O===4Fe(OH)3(����)��8OH����3O2�������ɵ������������壬���нϴ�ı���������������ʶ��ﵽ��ˮ��Ŀ��
��10H2O===4Fe(OH)3(����)��8OH����3O2�������ɵ������������壬���нϴ�ı���������������ʶ��ﵽ��ˮ��Ŀ��


 ����������������ϵ�д�
����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧ��ȡ9 g��������ˮ���ⶨ���۵�ˮ��ٷ��ʡ���������£�
������Һ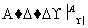 ����
����
����C��Һ�D��ש��ɫ����D
(1)����������Լ�Ϊ��
A________��B__________��C__________��
(2)����A��Һ��������B��Һ�Ƿ����________(����ԡ������ԡ�)����������________________________________________________________________________
________________________________________________________________________��
(3)������1.44 gש��ɫ���������۵�ˮ������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
G(�����ᱡ�ɴ���)��һ���������ಡ��ҩ���ϳ���·���£�
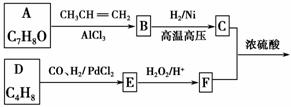
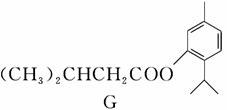
(1)A������Ϊ________��
(2)G�к�������������Ϊ________��
(3)D�ķ����к���________�ֲ�ͬ��ѧ��������ԭ�ӡ�
(4)E�����Ƶ�������ͭ��Ӧ�Ļ�ѧ����ʽΪ__________________________
__________________________________________________________��
(5)д����������������A������ͬ���칹��Ľṹ��ʽ��____________��
a����������6��̼ԭ����һ��ֱ���ϣ�
b���������OH��
(6)����ȩ���������ϡ��ٽ����ȣ�д�����Ҵ�Ϊԭ���Ʊ�CH3(CH2)3CHO�ĺϳ�·������ͼ(���Լ���ѡ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
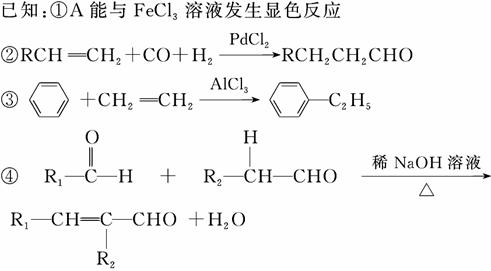
��һ��������ԭ��Ӧ��ϵ�з�Ӧ������ﹲ�������ӣ�Fe3����NO ��Fe2����NH
��Fe2����NH ��H����H2O������������ȷ���� (����)��
��H����H2O������������ȷ���� (����)��
A���÷�Ӧ˵��Fe(NO3)2��Һ���˼����ữ
B���÷�Ӧ���������뻹ԭ�����ʵ���֮��Ϊ8��1
C������1 mol NO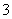 ����������Ӧ��ת�Ƶ���5 mol
����������Ӧ��ת�Ƶ���5 mol
D�������÷�Ӧ��Ƴ�ԭ��أ�����ӦΪFe3����e��===Fe2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ũ�ȹ�ϵ��ȷ����(����)
A����ˮ�У�c(Cl2)��2[c(ClO��)��c(Cl��)��c(HClO)]
B. ��ˮ�У�c(Cl��)>c(H��)>c(OH��)>c(ClO��)
C���������Ũ�ȵ���������������ϣ�c(Na��)��c(CH3COO��)
D��Na2CO3��Һ�У�c(Na��)>c(CO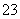 )>c(OH��)>c(HCO
)>c(OH��)>c(HCO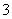 )>c(H��)
)>c(H��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ���Ʊ�BaCl2�Ĺ�������ͼ���£�
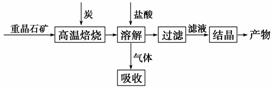
ij�о�С����ʵ�������ؾ�ʯ(��Ҫ�ɷ�BaSO4)�Թ�ҵ���̽���ģ��ʵ�顣����ã�
BaSO4(s)��4C(s)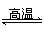
 4CO(g)��BaS(s)
4CO(g)��BaS(s)
��H1����571.2 kJ��mol��1����
BaSO4(s)��2C(s)
 2CO2(g)��BaS(s)
2CO2(g)��BaS(s)
��H2����226.2 kJ��mol��1����
(1)�����ù���NaOH��Һ���գ��õ����ơ���Na2Sˮ������ӷ���ʽΪ
________________________________________________________________________��
(2)��BaCl2��Һ�м���AgNO3��KBr�������ֳ�������ʱ��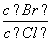 ��________��
��________��
[Ksp(AgBr)��5.4��10��13��Ksp(AgCl)��2.0��10��10]
(3)��Ӧ��C(s)��CO2(g)
 2CO(g)�Ħ�H��______kJ��mol��1��
2CO(g)�Ħ�H��______kJ��mol��1��
(4)ʵ�������б�����������̿��ͬʱ��Ҫͨ���������Ŀ����
________________________________________________________________________��
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������Ϸ��֣������Ʋ����ܸ�������ˮ��Ӧ�����ܸ������������ʷ�����Ӧ�����а�����ƾ��ڳ����·�Ӧ��Ҫ�о������Ƹ��ƾ���Ӧ�������Լ�����ˮ��Ӧ����ͬ �㣬���е��о�������û���õ����� �� ��
A��ʵ�鷨 B���۲취 C�����෨ D���ȽϷ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��CH4����ԭNOx���������������������Ⱦ�����磺
��CH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��g������H=��574kJ/mol
��CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2O��g������H=��1160kJ/mol
����˵���д�����ǣ� ��
A�������ʵ�����CH4�ڷ�Ӧ�١�����ת�Ƶ�������ͬ
B���ɷ�Ӧ�ٿ���֪��CH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��l������H>��574kJ/mol
C��4NO2��g��+2N2��g��=8NO��g������H=+586kJ/mol
D�����ñ�״����4.48L CH4��NO2��ԭΪN2������������ת�Ƶĵ�������Ϊ1.6NA
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com