
����Ŀ����������(CHBr2��CHBr2)��һ����ɫ��Һ�壬�ܶ�2.967g��mL-1��������ˮ���е�244�棬�������������ϵ���Ч�����ȡ��õ�ʯ(��Ҫ�ɷ�CaC2������CaS��Ca3P2��Ca3AS2��)��Br2��Ϊԭ���Ʊ��������������װ��(�г�װ����ʡ��)��ͼ1��ʾ��
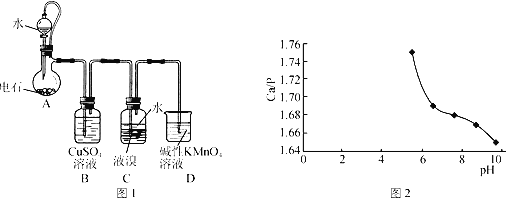
��1��װ��A��CaC2����ˮ���ҷ�����Ӧ��CaC2+2H2O��Ca(OH)2+C2H2����Ϊ�˵õ�ƽ����C2H2�����������ñ���ʳ��ˮ����ˮ�⣬���ɲ�ȡ�IJ���������__��
��2��װ��B�ɳ�ȥH2S��PH3��AsH3�����г�ȥPH3�Ļ�ѧ����ʽΪ__(PH3��ԭ�Խ�ǿ����������������)��
��3��װ��C����Һ��Һ���ϼ���һ��ˮ��Ŀ����__��װ��C�з�Ӧ����ɵ�������__����װ��C��Ӧ�����ϵ�õ���������Ʒ����Ҫ���еIJ�����__��
��4��һ���Ʊ�Ca10(PO4)6(OH)2��ԭ��Ϊ10Ca(OH)2+6H3PO4=Ca10(PO4)6(OH)2��+18H2O���������װ��A�õ���ʯ�����Ϊԭ���Ʊ�Ca10(PO4)6(OH)2��ʵ�鷽�������ձ��м���0.25L��0.5mol��L-1Ca(OH)2��ʯ���飬_����100������к��1h��
��֪��
��Ca10(PO4)6(OH)2��Ca/P������ֵΪ1.67��Ӱ���ƷCa/P�ȵ���Ҫ�����з�Ӧ��Ͷ�ϱȼ���ӦҺpH��
����95�棬pH��Ca/P�ȵ�Ӱ����ͼ2��ʾ��
��ʵ������ʹ�õ��Լ�����0.5mol��L-1Ca(OH)2��ʯ���顢0.3mol��L-1���ἰ����ˮ��
���𰸡���μ���(����ʳ��)ˮ 4CuSO4+PH3+4H2O=4Cu��+H3PO4+4H2SO4 ˮ�⣬����Һ��Ļӷ� ��������Һ�����Ϊ��ɫ���Ҽ�������������Ȳ�� ��Һ���л������������ռ�244����� �ڷ�Һ©���м���0.25L0.3mol��L-1���ᣬ��ʯ������ȵ�95�棬�ڲ��Ͻ����£��ȿ��ٵμ����ᣬȻ�������μӣ���ʱ�μ�����ˮ�Բ�����������ˮ�֣�ֱ������ȫ�����꣬���ڲ�������ҺpH8��9���ٳ�ֽ���һ��ʱ�䡢���ã����ˡ�ˮϴ
��������
A�е�ʯ��ˮ��Ӧ������Ȳ���������ƣ���Ϊ��ʯ�к���CaC2������CaS��Ca3P2��Ca3AS2�ȣ����Եõ�����Ȳ�к���H2S��PH3��AsH3��ԭ�����壬ͨ������ͭ��Һ����CuS��Cu��H2SO4��H3PO4��H3AsO4���Ӷ���ȥ��H2S��PH3��AsH3����ͨ��Һ�壬Һ�����Ȳ��Ӧ����1��1��2��2�������飬���������Һ��������ԭ�����塣
(1)Ϊ�˵õ�ƽ����C2H2�����������ñ���ʳ��ˮ����ˮ�⣬������ͨ���ı���뱥��ʳ��ˮ���ʿ��ƣ����Ըı�Ĵ�ʩΪ��μ���(����ʳ��)ˮ��
(2)������Ϣ֪�����������ͭ��Ӧ����Cu����������ᣬ��Ӧ����ʽΪ4CuSO4+PH3+4H2O=4Cu��+H3PO4+4H2SO4��
(3)���ж����ӷ���Ϊ��ֹ��ӷ�����Һ��ķ������岻������ˮ�����Բ���ˮ��ķ����������Ȳ��Ӧ����������ˮ��1��1��2��2���������ʹ����ɫ���������ɵ�1��1��2��2���������ˮ�ֲ㣬����������Һ�����Ϊ��ɫ���Ҽ�������������Ȳ��ʱ˵��C�з�Ӧ�Ѿ���ɣ��������ܵ�Һ����÷�Һ�������룬���ܵ�Һ������������룬����ˮ����л�����÷�Һ�������룬�л�����Һ����������������룬�ռ�244����֣�
(4)������Ϣ֪���÷�Ӧ��Ҫ95���������ʯ�����Ʊ�Ca10(PO4)6(OH)2��Һ����������ڷ�Һ©�����������٣�Ϊ�ӿ췴Ӧ������Ҫ���Ͻ��裬�ȿ��ٵμ����ᣬȻ�������μӣ�Ϊ��ֹ��Һ��ˮ�ּ��٣���Ҫ��ʱ�μ�����ˮ������������ˮ�֣�ֱ������ȫ�����꣬�ҿ�����Һ��pH��8��9���ٳ�ֽ���һ��ʱ�䡢���ã����ˡ�ˮϴ����100�������к��1 h���Ӷ��õ��ϸ����Ca10(PO4)6(OH)2.



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������־������־�����������й��ڰ�ͭ�ļ��أ���������ͭ��ͭ���Ͻ��������⣬����Ҫ������ң������������������Ʒ���ش��������⣺
��1����Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ______��3d�ܼ��ϵ�δ�ɶԵ�����Ϊ_______��
��2�����������ڰ�ˮ�γ�[Ni(NH3)6]SO4��ɫ��Һ��
��[Ni(NH3)6]SO4�������ӵ����幹����________��
����[Ni(NH3)6]SO4��Ni2+��NH3֮���γɵĻ�ѧ����Ϊ______���ṩ�µ��ӶԵijɼ�ԭ����______��
�۰��ķе�_______������ڡ����ڡ���좣�PH3����ԭ����_______������______���ӣ�����ԡ��Ǽ��ԡ���������ԭ�ӵĹ���ӻ�����Ϊ_______��
��3��ͭ����̼ͭԭ�ӵĶѻ���ʽ��ͼ��ʾ��
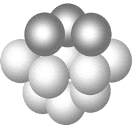
�ٻ�̬ͭ��Ԫ�����ڱ���λ��__________________��
��ÿ��ͭԭ����Χ���������ͭԭ����Ŀ_________��
(4)ijMԭ�ӵ���Χ�����Ų�ʽΪ3s23p5��ͭ��M�γɻ�����ľ�����ͼ��ʾ(�ڵ����ͭԭ��)��
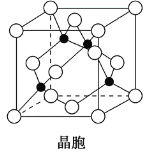
�ٸþ���Ļ�ѧʽΪ__________________��
����֪ͭ��M�ĵ縺�Էֱ�Ϊ1.9��3.0����ͭ��M�γɵĻ���������________(����ӡ����ۡ�)�����
����֪�þ�����ܶ�Ϊ�� g��cm��3�������ӵ�����ΪNA����þ�����ͭԭ�Ӻ�Mԭ��֮�����̾���Ϊ________pm(ֻд����ʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڼ��������ܷų���������ɫ��Һ�У��ֱ�������и������ӣ����ܴ����������
A.Ca2+��![]() ��
��![]() ��Na+B.Na+��Mg2+��
��Na+B.Na+��Mg2+��![]()
C.![]() ��Cu2+��K+��Cl��D.
��Cu2+��K+��Cl��D.![]() ��K+��Ba2+��OH��
��K+��Ba2+��OH��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͭ�����ճ������г����Ľ��������Ź㷺����;����ش��������⣺
(1)�����Fe(CO)x�����³�Һ̬���۵�Ϊ-20.5�棬�е�Ϊ103�棬�����ڷǼ����ܼ����ݴ˿��ж�Fe(CO)x��������__________(�������)��Fe(CO)x������ԭ�Ӽ۵������������ṩ������֮��Ϊ18����x=_____________��![]() �ĺ�������Ų�ʽΪ_____________________��
�ĺ�������Ų�ʽΪ_____________________��
(2)![]() ��Һ�����ڼ���_________(�����ӷ���)��
��Һ�����ڼ���_________(�����ӷ���)��![]() ��̼ԭ���ӻ��������Ϊ_____��1mol
��̼ԭ���ӻ��������Ϊ_____��1mol![]() ���еĦм���ĿΪ_______(��N��ʾ)��C��N��O��һ�������ɴ�С��˳��Ϊ_________(��Ԫ�ط��ű�ʾ)��
���еĦм���ĿΪ_______(��N��ʾ)��C��N��O��һ�������ɴ�С��˳��Ϊ_________(��Ԫ�ط��ű�ʾ)��
(3)ijMԭ�ӵ���Χ�����Ų�ʽΪ![]() ��ͭ��M�γɵ�ij������ľ����ṹ����ͼ��ʾ(�ڵ����ͭԭ��)��
��ͭ��M�γɵ�ij������ľ����ṹ����ͼ��ʾ(�ڵ����ͭԭ��)��
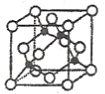
�ٸþ���Ļ�ѧʽΪ__________________��
����֪ͭ��M�ĵ縺�Էֱ�Ϊ1.9��3.0����ͭ��M�γɵĻ���������___________(����ӡ����ۡ�)�����
����֪�þ�����ܶ�Ϊ![]() �������ӵ�����Ϊ
�������ӵ�����Ϊ![]() ����þ�����ͭԭ�Ӻ�Mԭ��֮�����̾���Ϊ____________________pm(ֻ��д������ʽ)��
����þ�����ͭԭ�Ӻ�Mԭ��֮�����̾���Ϊ____________________pm(ֻ��д������ʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ؾ������õĿ��������ԡ������ǰ����ص�һ�ֺϳ�·�ߣ�

��֪����.�����������䣬ͨ����Ӧ��Ũ��Խ������Խ�죬�ಽ��Ӧʱ������������Ӧ������
��.�Ҷ�˹�����·�Ӧ��![]()
��1���л�������A�ķ���ʽΪ__��
��2��Aת��ΪBʱ��·�ߢ��·�ߢ����ˮ��Һ�н��С�ʵ�鷢�֣�·�ߢ��·�ߢ�����ʱ��̣��Խ���ԭ��__��
��3��д��B��C�Ļ�ѧ����ʽ__��
��4��D���Ҷ����������۷�Ӧ�Ļ�ѧ����ʽΪ__��
��5����X�Ľṹ��ʽ��__��
���±��о���__�Ժϳ�F��Ӱ�죬�ϳ�F�Ľ�������Ϊ__�����ţ���
��� | ����Һ��X | ��Ӧ�¶�/�� | ��Ӧʱ��/h | E����/% | F����/% | |
1 | [BPy]BF4 | 45 | 20 | 90 | 9 | |
2 | [HMIM]PF6 | 35 | 20 | 5 | 93 | |
3 | [BMIM]BF4 | 25 | 30 | 0 | 90 | |
4 | [HMIM]BF4 | 35 | 20 | 38 | 57 | |
5 | [BMIM]BF4 | 25 | 20 | 0 | 86 |
��6����д����![]() Ϊԭ���Ʊ�
Ϊԭ���Ʊ� �ĺϳ�·������ͼ__�����Լ���ѡ���ϳ�·������ͼʾ��������ɣ���
�ĺϳ�·������ͼ__�����Լ���ѡ���ϳ�·������ͼʾ��������ɣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͬ������,����X��ת��ΪY,Ҳ��ת��ΪZ,ת�������е������仯��ͼ��ʾ������˵����ȷ����(�� )
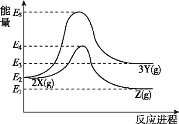
A.��2X��3Y�ķ�Ӧ�Ƿ��ȷ�Ӧ
B.X��Y��Z������������ȶ�����Z
C.��Ӧ���ܼ��ܴ���Z���ʵ��ܼ���
D.��Y��ת��ΪZ,����Ӧ�ķ�Ӧ�Ƿ��ȷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����ȥNaCO3��ĩ�л����NaHCO3������__________��������ѧ����ʽΪ____��
��2����ȥ����ͭ��ĩ�л������������ĩͨ����________�Լ������ӷ���ʽΪ________��
��3��ͨ����________�Լ���ȥCO2�е�HCl���壬���ӷ���ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������һ����Ҫ�Ļ���ԭ�ϣ���������������������Ƽ���ķ����У�������ǣ� ��
A.����Ӧ��ԭ����ͬB.������̼��Դ��ͬ
C.���а���ѭ������D.������һ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������װ�����Դﵽ��Ӧʵ��Ŀ�ĵ��ǣ� ��
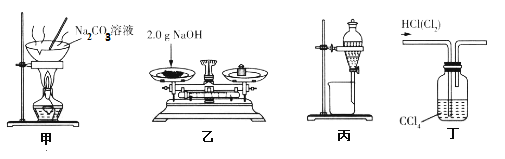
A.װ�üף������ᾧ���Ʊ�����
B.װ���ң�����������480 mL0. 10 mol��L-1 NaOH��Һ�������2.0 g NaOH
C.װ�ñ�����Һ�����Ȼ�̼��ȡ��ˮ�е�I2��Һ��õ�����Ȼ�̼��Һ
D.װ�ö���ϴ������ȥHCl��Cl2����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com