
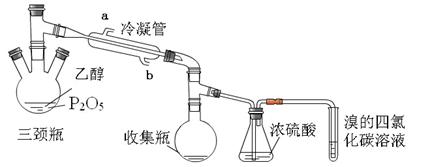
��ѧ����������������Ϊ�Ҵ���ˮ����ϩ�Ĵ������������ʵ�顣���ұ���ʾ�����ͷ�Ӧ����������ƿ�м���һ����P2O5����ע��95%���Ҵ��������ȣ��۲�����
| ʵ�� | P2O5 /g | 95%�Ҵ���/ mL | ���ȷ�ʽ |
| ʵ��1 | 2 | 4 | �ƾ��� |
| ʵ��2 | 2 | 4 | ˮԡ70�� |
| ʵ�� | ʵ������ | ||
| ����ƿ | �ռ�ƿ | �Թ� | |
| ʵ��1 | �ƾ�����ʱ�����̷ų������İ�������ʼ�����ݲ��������þƾ��Ƽ���ʱ�����ݼӿ����ɲ����ڣ�����ճ��״Һ�塣 | ����ɫҺ�� | ��Һ��ɫ |
| ʵ��2 | �ƾ�����ʱ���������������ɣ�����ˮԡ����ʱ�����������ݣ���Ӧһ��Сʱ����Ӧƿ������ճ��״Һ�� | ����ɫҺ�� | ��Һ����ɫ |
�����12�֣�ÿ��1��,38��2�֣�39�ڶ���2�֣�
��1��


��2������������ b����ˮ
��3����ϩ
��4�����ձ����¶ȼ�
��5�� ����Ӧ��ʹ���
����Ӧ��ʹ���
��6���ٽϸ��¶��»�ֱ�Ӽ��ȡ���������Ӧ��
���������������1���Ҵ���P2O5�����������·�����ȥ��Ӧ������ϩ��ˮ��
��2���������������͵��������ã�ˮ�������������෴������Ч���ã�����Y���·���bΪ��ˮ�ڣ����ɵ���ϩ�к���ˮ�֣�Ũ���������ˮ�����á�
��3����ϩ��Br2�����ӳɷ�Ӧ���Ӷ�ʹ������Ȼ�̼��Һ��ɫ��
��4��ˮ��Ҫ���ձ�ʢ�ţ�Ϊ�˲���ˮԡ���¶���Ҫ�¶ȼơ�
��5��P2O5��ˮ��Ӧ��������Ҵ�������ȥ��Ӧʱ��P2O5�������ã�P2O5�������ɵ�H2O��Ӧ����Ӧ������á�
��6���ٵ��þƾ��Ƽ���ʱ�����ݼӿ����ɲ����ڣ�����ճ��״Һ�壬˵�������ϩ�ķ�Ӧ�����ǽϸ��¶��»�ֱ�Ӽ��ȡ�
��P2O5��ˮ��Ӧ����H3PO4��H3PO4���Ҵ��ɷ���������Ӧ��
���㣺���⿼���л���ѧ����ʽ����д������������ʹ�ü����á��Լ������úͷ�Ӧ������ѡ��


 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����ֲ���纣���������к��зḻ�ĵ�Ԫ�أ���Ԫ���Ե����ӵ���ʽ���ڡ�ʵ������Ӻ�������ȡ����������£�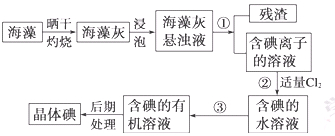
��1��ָ�������۵����ƣ� ���������г�������Cl2��Ŀ���� ��
��2����ȡ��Ĺ����У��ɹ�ѡ����Լ��� ( )
| A���ƾ� | B�����Ȼ�̼ | C������ | D������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijС�������ͼװ�ã����ּг�װ������ȥ������̽����ʪ��Cl2��Na2CO3��Ӧ�õ��������ʵijɷ֡�
��1���Լ�X�Ļ�ѧʽ ��A����ȡCl2�����ӷ���ʽ ��
��2���Լ�Y������Ϊ ��
��3��ͨ��һ������ʪ��Cl2��Ӧ����⣬D��ֻ��Cl2Oһ�����壬C��ֻ��һ�������⣬ͬʱ����NaHCO3�ȣ�ijͬѧ��C�����ù�������ijɷֽ���̽����
������������衣
����1���������ֳɷ֣�NaHCO3�� ��
����2���������ֳɷ֣�NaHCO3�� ��
����Ʒ���������ʵ�顣д��ʵ�鲽���Լ�Ԥ������ͽ��ۡ�
��ѡʵ���Լ�������������ˮ��ϡHNO3��BaCl2��Һ������ʯ��ˮ��AgNO3��Һ���Թܡ�С�ձ���
| ʵ�鲽�� | Ԥ������ͽ��� |
 ����1��ȡC�е�����������Ʒ���Թ��У��μ���������ˮ�������ܽ⣬Ȼ��������Һ�ֱ�����A��B�Թ��С� ����1��ȡC�е�����������Ʒ���Թ��У��μ���������ˮ�������ܽ⣬Ȼ��������Һ�ֱ�����A��B�Թ��С� | |
| ����2����A�Թ��еμ�BaCl2��Һ | |
| ����3�� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
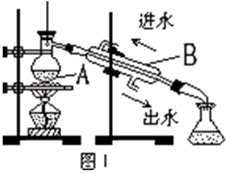
������һ����Ҫ�Ļ���ԭ�ϡ�ijѧϰС����ʵ������������ͼ��ʾװ����ȡ������̽�������ʡ�
��1��ʵ�����ö������̺�Ũ���������ȡ����������װ���г�Բ����ƿ�͵�������õ��IJ���������
��
��2��װ��A��ʢ�е��Լ��� �������� ��
��3����D��Ʒ����Һ��ɫ����Bװ���з�����Ӧ�����ӷ���ʽ��
��4��֤��FeBr2��Cl2������������ԭ��Ӧ��ʵ�鷽���� ���������������
ij�о���ѧϰС���ø����չ�����SO2��NaOH��Һ���մ�������ʵ����β��������������β��һ��ʱ�������Һ����ǿ���ԣ��п϶�����Cl����OH����CO32- ��SO42�������ڿ��ܴ��ڵ����������ӣ��о�С���������3�ּ��衣����1��ֻ����SO32��������2��ֻ����ClO��������3���Ȳ�����SO32����Ҳ������ClO����
��5��ѧϰС���ж�ͬʱ����SO32����ClO���Dz����ܵ������� ��
38.����ѡ�����Լ������ʵ�鷽��������ʵ�飬��д��ʵ�鲽���Լ�Ԥ������ͽ��ۡ�
a��3 mol/L H2SO4
b��0.01 mol/L KMnO4
c��1 mol/L BaCl2��Һ
d�����۵⻯����Һ
e����̪��Һ
����һ��ȡ��������Һ���Թ��У��μ�3 mol/L H2SO4����Һ�����ԣ�Ȼ��������Һ��װ��A��B
���Թ��С�
���������A�Թ��еμ�����___________ (�����)������Һ_________________�������������
1������
����������B�Թ��еμ�����___________������ţ�������Һ_________________�������������2
������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��(Ti)����Ϊ21���ͽ�����ұ���ѵ���Ҫԭ���Ǻ�Fe2O3��������FeTiO3)���������������£�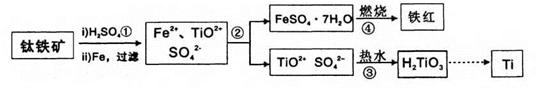
��֪��TiOSO4������ˮ����ˮ�⣬H2TiO3������ˮ���Իش��������⣺
(1)����ڵ���Ҫʵ���������ȴ���ᾧ�� (���������)���������ʵ�����г����Լ����� (���������ƣ��м�ǿ�ȡ�
(2)������м�����ˮ�������� ��
(3)�������������������(FeSO4��7H2O)�ڿ����������������졢ˮ����������д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
(4)��������õ���������������(FeSO4��7H2O)��Ŀǰ��ҵ�ϴ��������ж���ˮ���õĻ�ѧ�Լ������߷�Ӧ��(Cr��+6��ת��Ϊ+3��)����ת��Ϊ����Ҫ��ҵ��ֵ�������帴�����������FeO��FeyCrxO3��ʾ)�����Ʊ��������帴����������������Եĺ�����ˮ�У�����FeSO4��7H2O������ӦΪ��ˮ�����۸����൱��CrO3)������ ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧС��ͬѧΪ����֤�ճ��������û��ͷ�еĻ�ѧ�ɷ֣���KClO3��MnO2��S�ȣ������������ʵ�����̣���ͼ-1���� 
�Իش��������⣺
��1��ȼ�ŵĻ��ͼ-2����ʵ�飬���Թ����ܹ۲쵽 ���������֤�����ͷ�к�����Ԫ�ء�ͼ����Ͳ�������� ��
��2��Ϊ��֤�����ͷ�к�����Ԫ�أ�������ʵ�鲽���� ��
��3����ͬѧ���������ͷ��KClO3����һʵ�鷽����
�Լ�AΪ ������NaNO2��Ŀ���� ��
��4�����ʵ�飺������֤����D�к���MnO2��һ��ʵ�鷽������д���йط�Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��(Sr)Ϊ�������ڢ�A��Ԫ�ء��ߴ���ˮ�Ȼ��Ⱦ���(SrCl2?6H2O)���кܸߵľ��ü�ֵ��61��ʱ���忪ʼʧȥ�ᾧˮ��100��ʱʧȥȫ���ᾧˮ���ù�ҵ̼���ȷ�ĩ(������Ba��Fe�Ļ�����)�Ʊ��ߴ���ˮ�Ȼ��ȵĹ�������ͼ��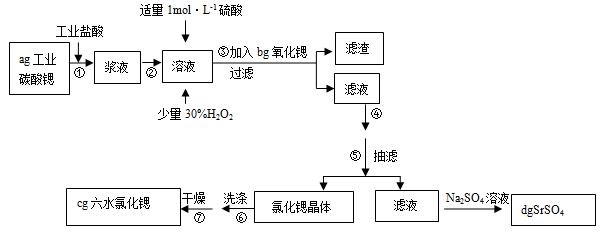
��ش�
��1����������30%H2O2������ �������ӷ���ʽ��ʾ����
��2��������������ȷ�ĩ�������� ����ҵ����50��60���ȷ紵����ˮ�Ȼ��ȣ�ѡ����¶ȵ�ԭ���� ��
��3������ܽ��е�ʵ�����Ϊ �� ��������У�ϴ���Ȼ��Ⱦ������ѡ�� ��
| A��ˮ | B������ | C������������Һ | D���Ȼ��ȱ�����Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�����������ų������ı���[��Ҫ��BaCO3��BaSO3��Ba(FeO2)2��]��ij��Ҫ����BaCO3�Ļ��������ñ�����ȡBa(NO3)2���弰����������䲿�ֹ����������£�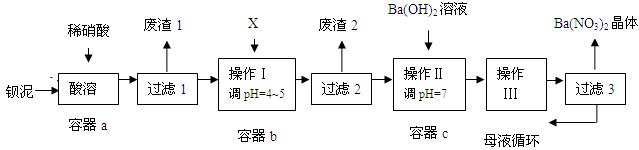
��֪���� Fe(OH)3��Fe(OH)2��ȫ����ʱ����Һ��pH�ֱ�Ϊ3.2��9.7
�� Ba(NO3)2����ˮ���ܽ�Ƚϴ�����ˮ���ܽ�Ƚ�С
�� KSP(BaSO4)=1.1��10��10��KSP (BaCO3)=5.1��10��9
��1���ó�������BaCO3��������BaSO4���������ᴿ�ķ����ǣ�����Ʒ���������ı���Na2CO3��Һ�У���ֽ��裬���ˣ�ϴ�ӡ������ӷ���ʽ˵���ᴿԭ���� ��
��2��������������ʱ��Ba(FeO2)2��HNO3��Ӧ�������������Σ���ѧ����ʽΪ��
��
��3���ó���ϱ���ʵ�ʣ�ѡ�õ�XΪ ��
| A��BaCl2 | B��BaCO3 | C��Ba(NO3)2 | D��Ba(OH)2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
������ˮԡ���ȵ�ʵ���ǣ� ��
| A������������Ӧ | B��������Ӧ | C���Ʒ�ȩ��֬ | D�����Ҵ�����ϩ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com