
 ��t��ʱ�����ݻ�Ϊ2L���ܱ��������м���1mol A���塢3mol B���弰��������������������з�Ӧ��A��g��+3B��g��?2C��g������H��0��10min������ʵ�Ũ�Ȳ��ٱ仯���������C���������Ϊ25%������գ�
��t��ʱ�����ݻ�Ϊ2L���ܱ��������м���1mol A���塢3mol B���弰��������������������з�Ӧ��A��g��+3B��g��?2C��g������H��0��10min������ʵ�Ũ�Ȳ��ٱ仯���������C���������Ϊ25%������գ�| 2x |
| 4-2x |
| ||
| 10min |
| 0.4mol |
| 1mol |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(14 ��)��T1��ʱ�����ݻ�Ϊ2 L ���ܱ��������м���1mol N1��3mol H2����������� ������������ӦN2(g) + 3H2(g) ![]() 2NH3(g)����H��0��10minʱ�����ʵ�Ũ�Ȳ��ٱ仯�����NH3 ���������Ϊ25% ��
2NH3(g)����H��0��10minʱ�����ʵ�Ũ�Ȳ��ٱ仯�����NH3 ���������Ϊ25% ��
(1) �÷�Ӧ��0 ~l0min ʱ����H2��ƽ����Ӧ����Ϊ______��N2��ת����Ϊ______��
 (2)��T1��ʱ������ʼʱ���������м���0.5mol N2��1.5mol H2��0.5mol NH3 ����ﵽƽ��ʱNH3���������______ (��ѡ����ĸ) ��
(2)��T1��ʱ������ʼʱ���������м���0.5mol N2��1.5mol H2��0.5mol NH3 ����ﵽƽ��ʱNH3���������______ (��ѡ����ĸ) ��
a.����25% b.����25% c.��25%
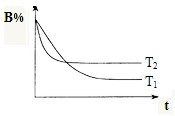
(3)��ͼ����T1��ʱ�ܱ���������H2�����������ʱ��t�ı仯���ߣ����ڸ�ͼ�в������÷�Ӧ��T2�棨T1 ��T2��ʱH2�����������ʱ��t�ı仯���ߡ�
(4)������T1�棬�����ҲΪ2 L ���ܱ���������ͨ��һ������N2��H2��NH3����ʹƽ��ʱ�������и����ʵ����ʵ���������������ȫ��ͬ������ʼʱ��Ӧ������Ӧ������У���ͨ��H2�����ʵ���x��ȡֵ��Χ��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ʯ��ׯ�и��б�ҵ�ิϰ��ѧ�������������ۺ��������Ի�ѧ���� ���ͣ������
��T1��ʱ�����ݻ�Ϊ2 L ���ܱ��������м���1mol N1��3mol H2����������� ������������ӦN2(g) + 3H2(g)  2NH3(g)����H��0��10minʱ�����ʵ�Ũ�Ȳ��ٱ仯�����NH3 ���������Ϊ25% ��
2NH3(g)����H��0��10minʱ�����ʵ�Ũ�Ȳ��ٱ仯�����NH3 ���������Ϊ25% ��
(1) �÷�Ӧ��0 ~l0min ʱ����H2��ƽ����Ӧ����Ϊ______��N2��ת����Ϊ______�� (2)��T1��ʱ������ʼʱ���������м���0.5mol N2��1.5mol H2��0.5mol NH3 ����ﵽƽ��ʱNH3���������______ (��ѡ����ĸ) ��
(2)��T1��ʱ������ʼʱ���������м���0.5mol N2��1.5mol H2��0.5mol NH3 ����ﵽƽ��ʱNH3���������______ (��ѡ����ĸ) ��
a.����25% b.����25% c.��25%
(3)��ͼ����T1��ʱ�ܱ���������H2�����������ʱ��t�ı仯���ߣ����ڸ�ͼ�в������÷�Ӧ��T2�棨T1��T2��ʱH2�����������ʱ��t�ı仯���ߡ�
(4)������T1�棬�����ҲΪ2 L ���ܱ���������ͨ��һ������N2��H2��NH3����ʹƽ��ʱ�������и����ʵ����ʵ���������������ȫ��ͬ������ʼʱ��Ӧ������Ӧ������У���ͨ��H2�����ʵ���x��ȡֵ��Χ��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡģ���� ���ͣ������
 2C(g)����H
2C(g)����H 
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��T1��ʱ�����ݻ�Ϊ2 L ���ܱ��������м���1mol N1��3mol H2����������� ������������ӦN2(g) + 3H2(g) ![]() 2NH3(g)����H��0��10minʱ�����ʵ�Ũ�Ȳ��ٱ仯�����NH3 ���������Ϊ25% ��
2NH3(g)����H��0��10minʱ�����ʵ�Ũ�Ȳ��ٱ仯�����NH3 ���������Ϊ25% ��
(1) �÷�Ӧ��0 ~l0min ʱ����H2��ƽ����Ӧ����Ϊ______��N2��ת����Ϊ______��
 (2)��T1��ʱ������ʼʱ���������м���0.5mol N2��1.5mol H2��0.5mol NH3 ����ﵽƽ��ʱNH3���������______ (��ѡ����ĸ) ��
(2)��T1��ʱ������ʼʱ���������м���0.5mol N2��1.5mol H2��0.5mol NH3 ����ﵽƽ��ʱNH3���������______ (��ѡ����ĸ) ��
a.����25% b.����25% c.��25%
(3)��ͼ����T1��ʱ�ܱ���������H2�����������ʱ��t�ı仯���ߣ����ڸ�ͼ�в������÷�Ӧ��T2�棨T1 ��T2��ʱH2�����������ʱ��t�ı仯���ߡ�
(4)������T1�棬�����ҲΪ2 L ���ܱ���������ͨ��һ������N2��H2��NH3����ʹƽ��ʱ�������и����ʵ����ʵ���������������ȫ��ͬ������ʼʱ��Ӧ������Ӧ������У���ͨ��H2�����ʵ���x��ȡֵ��Χ��____________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com