
Ρ≥―ß…ζ…ηΦΤ“ΜΗω Β―ι÷ΛΟςPbO÷–Κ§”–―θΘ§Ζ¥”ΠΖΫ≥Χ Ϋ»γœ¬ΘΚ
PbO+C![]() Pb+CO(÷ς“Σ)ΘΜPbO+CO
Pb+CO(÷ς“Σ)ΘΜPbO+CO![]() Pb+
Pb+![]() ΘΜ
ΘΜ
[Cu(NH3)2]Ac+CO+NH3![]() [Cu(NH3)3]AcΓΛCO +QkJ
[Cu(NH3)3]AcΓΛCO +QkJ
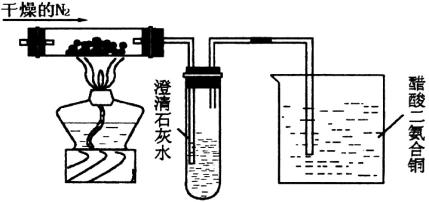
ΜΊ¥πœ¬Ν–”–ΙΊΈ ΧβΘΚ
(1) Δ≥Έ«ε ·Μ“Υ°ΒΡ ‘ΙήΩΣ Φ“ΜΕΈ ±ΦδΩ…ΡήΟΜ”–œ÷œσΘ§Τδ‘≠“ρ «________ΓΘ
(2)…’±≠÷–¥ΉΥαΕΰΑ±ΚœΆ≠([Cu(NH3)2]ΓΛAc)ΒΡΉς”ΟΈΣ________ΓΘ
(3) Β―ιΚσΒΡ¥ΉΥαΕΰΑ±ΚœΆ≠Ψ≠Ιΐ Β±¥Πάμ”÷Ω…‘Ό…ζΘ§ “Υ”Ύ‘Ό…ζΒΡ…ζ≤ζΧθΦΰ «________ΓΘ
(4)»τ”ΟΩ’Τχ¥ζΧφΗ…‘οN2Θ§––¬πΘΩ________Θ§Τδάμ”… «________ΓΘ

| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚΈοάμΫΧ―– “ Χβ–ΆΘΚ022
PbO+C![]() Pb+CO(÷ς“Σ)ΘΜPbO+CO
Pb+CO(÷ς“Σ)ΘΜPbO+CO![]() Pb+
Pb+![]() ΘΜ
ΘΜ
[Cu(NH3)2]Ac+CO+NH3![]() [Cu(NH3)3]AcΓΛCO +QkJ
[Cu(NH3)3]AcΓΛCO +QkJ
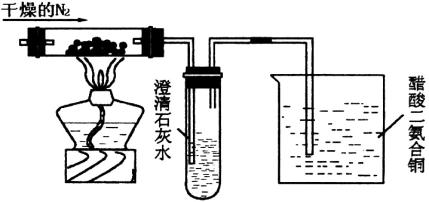
ΜΊ¥πœ¬Ν–”–ΙΊΈ ΧβΘΚ
(1) Δ≥Έ«ε ·Μ“Υ°ΒΡ ‘ΙήΩΣ Φ“ΜΕΈ ±ΦδΩ…ΡήΟΜ”–œ÷œσΘ§Τδ‘≠“ρ «________ΓΘ
(2)…’±≠÷–¥ΉΥαΕΰΑ±ΚœΆ≠([Cu(NH3)2]ΓΛAc)ΒΡΉς”ΟΈΣ________ΓΘ
(3) Β―ιΚσΒΡ¥ΉΥαΕΰΑ±ΚœΆ≠Ψ≠Ιΐ Β±¥Πάμ”÷Ω…‘Ό…ζΘ§ “Υ”Ύ‘Ό…ζΒΡ…ζ≤ζΧθΦΰ «________ΓΘ
(4)»τ”ΟΩ’Τχ¥ζΧφΗ…‘οN2Θ§––¬πΘΩ________Θ§Τδάμ”… «________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚΈοάμΫΧ―– “ Χβ–ΆΘΚ058

PbO+C![]() Pb+COΘ®÷ς“ΣΘ©
Pb+COΘ®÷ς“ΣΘ©
PbO+CO![]() Pb+CO2
Pb+CO2
[Cu(NH3)2]Ac+CO+NH3ƒ[Cu(NH3)3]AcΓΝCO+QkJ
ΜΊ¥πœ¬Ν–”–ΙΊΈ ΧβΘΚ
ΔΌ Δ≥Έ«ε ·Μ“Υ°ΒΡ ‘ΙήΩΣ Φ“ΜΕΈ ±ΦδΩ…ΡήΟΜ”–œ÷œσΘ§Τδ‘≠“ρ «________ΓΘ
ΔΎ…’±≠άο¥ΉΥαΕΰΑ±ΚœΆ≠([Cu(NH3)2]ΓΝAc)ΒΡΉς”ΟΈΣ________________ΓΘ
Δέ»τ”ΟΩ’Τχ¥ζΧφΗ…‘οN2––¬πΘΩ________Τδάμ”… «________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ058
»γΆΦΘ§Ρ≥―ß…ζ…ηΦΤ“ΜΗω Β―ι÷ΛΟςPbO÷–Κ§”–―θΘ§Ζ¥”ΠΖΫ≥Χ Ϋ»γœ¬ΘΚ

PbO+C![]() Pb+COΘ®÷ς“ΣΘ©
Pb+COΘ®÷ς“ΣΘ©
PbO+CO![]() Pb+CO2
Pb+CO2
[Cu(NH3)2]Ac+CO+NH3ƒ[Cu(NH3)3]AcΓΝCO+QkJ
ΜΊ¥πœ¬Ν–”–ΙΊΈ ΧβΘΚ
ΔΌ Δ≥Έ«ε ·Μ“Υ°ΒΡ ‘ΙήΩΣ Φ“ΜΕΈ ±ΦδΩ…ΡήΟΜ”–œ÷œσΘ§Τδ‘≠“ρ «________ΓΘ
ΔΎ…’±≠άο¥ΉΥαΕΰΑ±ΚœΆ≠([Cu(NH3)2]ΓΝAc)ΒΡΉς”ΟΈΣ________________ΓΘ
Δέ»τ”ΟΩ’Τχ¥ζΧφΗ…‘οN2––¬πΘΩ________Τδάμ”… «________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
Ρ≥―ß…ζ…ηΦΤ“ΜΗω Β―ι÷ΛΟςPbO÷–Κ§”–―θ‘ΣΥΊΘ§Ζ¥”Π Ϋ»γœ¬ΘΚ
PbO+C ![]() Pb+COΓϋΘ§
Pb+COΓϋΘ§
PbO+CO![]() Pb+CO2
Pb+CO2
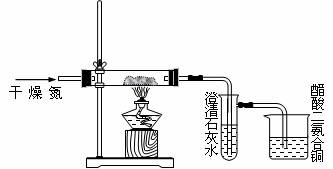
ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©¥ΥΉΑ÷ΟΆΦ÷–”–ΗωΟςœ‘ΒΡ¥μΈσΘ§«κ÷Η≥ωΓΘ
Θ®2Θ© Δ≥Έ«ε ·Μ“Υ°ΒΡ ‘ΙήΩΣ Φ“ΜΕΈ ±ΦδΩ…ΡήΟΜ”–œ÷œσΘ§«κ÷Η≥ωΩ…ΡήΒΡ‘≠“ρ
Θ®3Θ©…’±≠÷–¥ΉΥαΕΰΑ±ΚœΆ≠ΒΡΉς”Ο « ≤Ο¥ΘΩ
Θ®4Θ©»τ”ΟΩ’Τχ¥ζΧφΗ…‘οΒΡΒΣΤχΘ§––¬πΘΩΈΣ ≤Ο¥ΘΩ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com