
·ÖĪö £Ø1£©ŅĄ¾ŻĢāÖŠČČ»Æѧ·½³ĢŹ½ŗĶøĒĖ¹¶ØĀÉŠ“³ö±ūĶéĶŃĒāµĆ±ūĻ©µÄČČ»Æѧ·½³ĢŹ½£»
£Ø2£©øł¾ŻČČ»Æѧ·½³ĢŹ½µÄŹéŠ“·½·ØæÉÖŖ£¬ĪļÖŹµÄĪļÖŹµÄĮæÓė·“Ó¦·Å³öµÄČČĮæ³ÉÕż±Č£¬²¢×¢Ņā±źĆ÷ø÷ĪļÖŹµÄ¾Ū¼ÆדĢ¬Ą“½ā“š£»
ĻČøł¾ŻøĒĖ¹¶ØĀÉŠ“³ö·½³ĢŹ½£¬Č»ŗóøł¾ŻĪļÖŹµÄĪļÖŹµÄĮæÓė·“Ó¦·Å³öµÄČČĮæ³ÉÕż±ČĄ“½ā“š£®
½ā“š ½ā£ŗ£Ø1£©¢ŁC3H8£Øg£©”śCH4£Øg£©+HC”ŌCH£Øg£©+H2£Øg£©”÷H1=+156.6kJ•mol-1
¢ŚCH3CH=CH2£Øg£©”śCH4£Øg£©+HC”ŌCH£Øg£©”÷H2=+32.4kJ•mol-1
ŅĄ¾ŻøĒĖ¹¶ØĀÉ¢Ł-¢ŚæÉµĆ£ŗC3H8£Øg£©”śCH3CH=CH2£Øg£©+H2£Øg£©”÷H=+124.2KJ/mol£¬
¹Ź“š°øĪŖ£ŗC3H8£Øg£©”śCH3CH=CH2£Øg£©+H2£Øg£©”÷H=+124.2KJ/mol£»
£Ø2£©0.3molĘųĢ¬øßÄÜČ¼ĮĻŅŅÅšĶéŌŚŃõĘųÖŠČ¼ÉÕ£¬Éś³É¹ĢĢ¬ČżŃõ»Æ¶žÅšŗĶŅŗĢ¬Ė®£¬·Å³ö649.5KJµÄČČĮ棬Ōņ1molĘųĢ¬øßÄÜČ¼ĮĻŅŅÅšĶéŌŚŃõĘųÖŠČ¼ÉÕ£¬Éś³É¹ĢĢ¬ČżŃõ»Æ¶žÅšŗĶŅŗĢ¬Ė®£¬·Å³ö2165KJµÄČČĮ棬·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ£ŗB2H6£Øg£©+3O2£Øg£©=B2O3£Øs£©+3H2O£Øl£©”÷H=-2165kJ/mol£¬
øł¾Ż£ŗB2H6£Øg£©+3O2£Øg£©=B2O3£Øs£©+3H2O£Øl£©”÷H=-2165kJ/mol£» ¢Ł
H2O£Øl£©”śH2O£Øg£©£»”÷H=+44kJ/moL£¬¢Ś
¢Ł+¢Ś”Į3µĆ£ŗB2H6£Øg£©+3O2£Øg£©=B2O3£Øs£©+3H2O£Øg£©”÷H=-2033kJ/mol£¬¹Ź5.6L£Ø±ź×¼×“æö£©¼“0.25molŅŅÅšĶéĶźČ«Č¼ÉÕÉś³ÉĘųĢ¬Ė®Ź±·Å³öµÄČČĮæŹĒĪŖ2033kJ”Į0.25=508.25kJ£»
¹Ź“š°øĪŖ£ŗB2H6£Øg£©+3O2£Øg£©=B2O3£Øs£©+3H2O£Øl£©”÷H=-2165kJ/mol£»508.25£®
µćĘĄ ±¾ĢāÖ÷ŅŖæ¼²éĮĖČČ»Æѧ·½³ĢŹ½µÄŹéŠ“£¬øĒĖ¹¶ØĀɵÄŌĖÓĆŅŌ¼°ČČĮæµÄ¼ĘĖć£¬ŠčŅŖ×¢ŅāµÄŹĒ·“Ó¦ČȵďżÖµÓė»Æѧ·½³ĢŹ½Ē°ĆęµÄĻµŹż³ÉÕż±Č£¬ĢāÄæÄѶČÖŠµČ£¬²ąÖŲÓŚæ¼²éѧɜµÄ·ÖĪöÄÜĮ¦ŗĶ¼ĘĖćÄÜĮ¦£®


 ·¢É¢Ė¼Ī¬ŠĀæĪĢĆĻµĮŠ“š°ø
·¢É¢Ė¼Ī¬ŠĀæĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Al2£ØSO4£©3 | B£® | Na2CO3 | C£® | AlCl3 | D£® | KNO3 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŗĻ½š | B£® | ¹čĖįŃĪ²ÄĮĻ | C£® | ÓŠ»śøß·Ö×Ó²ÄĮĻ | D£® | ĪŽ»ś·Ē½šŹō²ÄĮĻ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
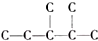 £¬“ĖĶéĢžµÄŅ»äå“śĪļÓŠ6 ÖÖ£¬Čō“ĖĶéĢžĪŖČ²Ģž¼ÓĒāÖĘµĆ£¬Ōņ“ĖČ²ĢžµÄ½į¹¹¼ņŹ½ĪŖ£ØCH3£©2CH£ØCH3£©CHC”ŌCH£®
£¬“ĖĶéĢžµÄŅ»äå“śĪļÓŠ6 ÖÖ£¬Čō“ĖĶéĢžĪŖČ²Ģž¼ÓĒāÖĘµĆ£¬Ōņ“ĖČ²ĢžµÄ½į¹¹¼ņŹ½ĪŖ£ØCH3£©2CH£ØCH3£©CHC”ŌCH£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| H2£Øg£© | Cl2 £Øg£© | HCl £Øg£© | |
| 1mol·Ö×ÓÖŠµÄ»Æѧ¼ü¶ĻĮŃŹ±ŠčŅŖĪüŹÕµÄÄÜĮæ/kJ | 436 | 243 | a |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | M£¼N | B£® | 2M=N | C£® | N£¼M | D£® | M=N |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | CO2”śCO | B£® | Zn”śZn2+ | C£® | H2”śH2O | D£® | CuO”śCuCl2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ClŌ×ӵĽį¹¹Ź¾ŅāĶ¼£ŗ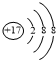 | B£® | “ĪĀČĖįµÄ½į¹¹Ź½£ŗH-Cl-O | ||
| C£® | NH4ClµÄµē×ÓŹ½£ŗ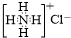 | D£® | ¼äĻõ»ł¼×±½µÄ½į¹¹¼ņŹ½£ŗ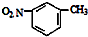 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com