
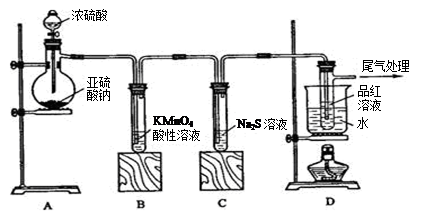

 £Ø2·Ö£¬Ęä֊װÖĆ1·Ö£¬±ź×¢ŹŌ¼Į1·Ö£¬×°ÖĆ”¢ŹŌ¼Į
£Ø2·Ö£¬Ęä֊װÖĆ1·Ö£¬±ź×¢ŹŌ¼Į1·Ö£¬×°ÖĆ”¢ŹŌ¼Į ”£2Öʱø”¢ŠŌÖŹŃéÖ¤”¢Ī²Ęų“¦ĄķŅŌ¼°Ąė×Ó¼ģŃé
”£2Öʱø”¢ŠŌÖŹŃéÖ¤”¢Ī²Ęų“¦ĄķŅŌ¼°Ąė×Ó¼ģŃé


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ŅŖĒåĻ“ø½×ÅŌŚŹŌ¹Ü±ŚÉĻµÄĮņ£¬æÉÓƵďŌ¼ĮŹĒCS2 |
| B£®Įņ»ÆŃĒĢśČÜÓŚĻ”ĻõĖįÖŠ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗFeS+2H+=Fe2++H2S”ü |
| C£®³£ĪĀĻĀÅØĮņĖįÄÜŹ¹ĀĮ·¢Éś¶Ū»Æ£¬æÉŌŚ³£ĪĀĻĀÓĆĀĮÖĘČŻĘ÷ÖüŌĖÅØĮņĖį |
| D£®ŌŚNa2SO3ŗĶBaCO3»ģŗĻĪļÖŠ¼ÓČė¹żĮæµÄĻ”ĮņĖį£¬ÄܲśÉśĘųÅŻ²¢ÓŠ³ĮµķÉś³É |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®±»ĖįÓźĪŪČ¾µÄÅ©Ģļ¼°ŗž²“æÉČ÷ŹÆ»ŅŹÆ·ŪÄ©£»¼õĒįĘäĪ£ŗ¦ |
| B£®ŌŚČÜŅŗBaCl2ÖŠĶØČėSO2ĘųĢ壬ČÜŅŗČŌµĒĒ壬µĪČė3%¹żŃõ»ÆĒāČÜŅŗÓŠ°×É«³Įµķ |
| C£®ÄÜÓėĖ®·“Ӧɜ³ÉĮņĖį |
| D£®¶žŃõ»ÆĮņÄÜŹ¹Ę·ŗģČÜŅŗĶŹÉ«£¬¼ÓČČ£¬Ę·ŗģČÜŅŗŃÕÉ«»Öø“ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®¶žŃõ»ÆĮņ¾ßÓŠĘư׊Ō£¬ĖłŅŌæÉŅŌŹ¹äåĖ®ĶŹÉ« |
| B£®ĮņŌŚÉŁĮæŃõĘųÖŠČ¼ÉÕÉś³É¶žŃõ»ÆĮņ£¬ŌŚ¹żĮæŃõĘųÖŠČ¼ÉÕÉś³ÉČżŃõ»ÆĮņ |
| C£®Ą×ÓźĢģµŖŌŖĖŲæÉŅŌ½ųŠŠĻĀĮŠ×Ŗ»Æ£ŗN2”śNO”śNO2”śHNO3 |
| D£®µŖĘųµÄ»ÆѧŠŌÖŹ·Ē³£ĪČ¶Ø£¬²»Ö§³ÖČĪŗĪĪļÖŹµÄČ¼ÉÕ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
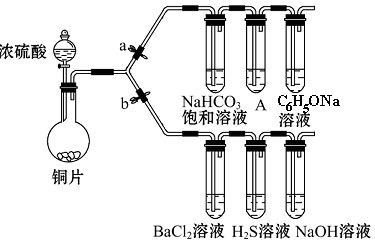
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ĶعżŹÆ»ŅĖ® | B£®ĻČĶعżĖįŠŌKMnO4ČÜŅŗŌŁĶعżŹÆ»ŅĖ® |
| C£®ĶعżĘ·ŗģČÜŅŗ | D£®ĻČĶعżŠ”ĖÕ“ņČÜŅŗŌŁĶعżŹÆ»ŅĖ® |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®Į½Õ߶¼Ņ×ČÜÓŚĖ® | B£®Į½Õ߶¼ĪŪČ¾»·¾³£¬Ī£ŗ¦½”æµ |
| C£®Į½Õ߶¼ÄÜŹ¹Ę·ŗģČÜŅŗĶŹÉ« | D£®Į½Õ߶¼ŹĒŠĪ³ÉĖįÓźµÄÖ÷ŅŖŌŅņ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗ¼ĘĖćĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com