
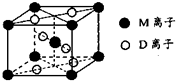
 ��2013?����ģ�⣩A��B��D��E��G��M�������ֳ���Ԫ�أ����ǵĺ˵���������������У�Ԫ��M�Ļ�̬3d�������2�����ӣ�A�Ļ�̬ԭ��L���������K���������2����E�� ��������ͬ����Ԫ�صļ������а뾶��С��D��Gͬ���壻B��D�γɵĻ������ж��֣�����һ���Ǻ���ɫ���壮
��2013?����ģ�⣩A��B��D��E��G��M�������ֳ���Ԫ�أ����ǵĺ˵���������������У�Ԫ��M�Ļ�̬3d�������2�����ӣ�A�Ļ�̬ԭ��L���������K���������2����E�� ��������ͬ����Ԫ�صļ������а뾶��С��D��Gͬ���壻B��D�γɵĻ������ж��֣�����һ���Ǻ���ɫ���壮| 1 |
| 2 |
| 1 |
| 8 |
| 1 |
| 2 |
| 8 |
| 3 |
8.4��
| ||
| 112 |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2013?����ģ�⣩ʵ������ʵ��Ŀ��װ�û������ȷ���ǣ�������
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2013?����ģ�⣩25��ʱ��c��CH3COOH��+c��CH3COO-��=0.1mol?L-1�Ĵ��ᡢ�����ƻ����Һ�У�c��CH3COOH����c��CH3COO-����pH�Ĺ�ϵ��ͼ��ʾ�������йظ���Һ����������ȷ���ǣ�������
��2013?����ģ�⣩25��ʱ��c��CH3COOH��+c��CH3COO-��=0.1mol?L-1�Ĵ��ᡢ�����ƻ����Һ�У�c��CH3COOH����c��CH3COO-����pH�Ĺ�ϵ��ͼ��ʾ�������йظ���Һ����������ȷ���ǣ��������鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com