


 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ͬŨ�ȡ�ͬ�����ǿ����ǿ����Һ��Ϻ���Һһ��Ϊ���� |
| B����Na2S��Һ�У�c��Na+��=2[c��S2-��+c��HS-��+c��H2S��] |
| C����1 mol KOH����Һ��1 mol CO2��ȫ��Ӧ����Һ��c��K+��=c��HCO3-�� |
| D����CH3COONa��Һ�м�������CH3COOH����ʹc��Na+��=c��CH3COO-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �¶�/�� | 800 | 1000 | 1200 | 1400 |
| ƽ�ⳣ�� | 0.45 | 1.92 | 276.5 | 1771.5 |
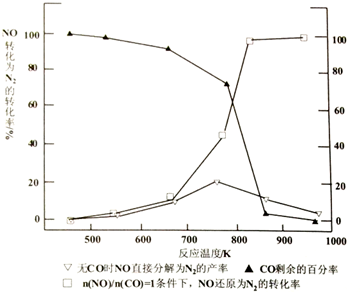
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | X | Y | Z |
| ��ʼŨ��/mol?L-1 | 0.1 | 0.2 | 0 |
| ƽ��Ũ��/mol?L-1 | 0.05 | 0.05 | 0.1 |
| A����Ӧ�ﵽƽ��ʱ��Y��ת����Ϊ75% |
| B���÷�Ӧ�ɱ�ʾΪX+3Y?2Z���ҳ����µ�ƽ�ⳣ��Ϊ1600 |
| C��������������ʱ������ѹǿ��ʹ����ƽ��������Z�ķ����ƶ�����ƽ�ⳣ�������� |
| D���������¶���ʹ�÷�Ӧ��ƽ�ⳣ����С��˵���÷�Ӧ������ӦΪ���ȷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����������7.04gͭ |
| B�������缫��ӦΪ��Cu2++2e-�TCu��2H++2e-�TH2�� |
| C�������缫��ӦΪ��4OH--4e-�T2H2O+O2�� |
| D����������Һ�м���ͭ����ܽ�7.04gͭ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com