
³¤ĘŚ“ę·ÅµÄNa2SO3»į±»æÕĘųÖŠµÄŃõĘų²æ·ÖŃõ»Æ£ŗij»ÆѧŠĖȤŠ”×éĶعżŹµŃé²ā¶ØijĪŽĖ®Na2SO3±»Ńõ»ÆµÄ³Ģ¶Č”£
£Ø¢ń£©¼×Ķ¬Ń§Éč¼ĘĮĖĻĀĶ¼ŹµŃé
Ēė»Ų“š£ŗ
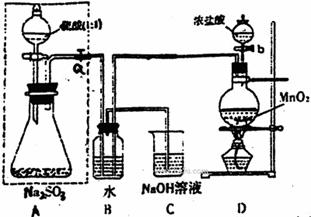
£Ø1£©Čō½«ŠéĻßæņÄŚµÄ·ÖŅŗĀ©¶·»»³É³¤¾±Ā©¶·£¬ŌņÓ¦ČēŗĪ¼ģ²éŠéĻßæņČװÖƵÄĘųĆÜŠŌ£æ
ӣ
£Ø2£©Š“³öB×°ÖĆÖŠµÄĄė×Ó·½³ĢŹ½ ”£
Š“³öD×°ÖĆÖŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
£Ø3£©³ĘĮæagNa2SO3ѳʷ·ÅČė׶ŠĪĘæÖŠ£¬ĻņB×°ÖĆ·“Ó¦ŗóµÄČÜŅŗÖŠ¼ÓČė×ćĮæBaCl2ČÜŅŗ
³ä·Ö·“Ó¦£¬¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ”¢³ĘĮæµĆ°×É«³Įµķbg£¬Ōņѳʷ֊Na2SO3µÄÖŹĮæ·ÖŹżĪŖ ”£
£Ø4£©ÉīČėŃŠ¾æ·¢ĻÖ£¬ÉĻŹöŹµŃé·½°ø“ęŌŚČ±ĻŻ£¬ÖĀŹ¹²āµĆµÄNa2SO3ÖŹĮæ·ÖŹżĘ«Š”£¬ŹŌ·ÖĪöĘäÖŠµÄŌŅņ£ØĮŠ³öŅ»Ģõ¼“æÉ£© ”£
 £Ø¢ņ£©ŅŅĶ¬Ń§Éč¼ĘĮĖĮķŅ»Ģ׏µŃé×°ÖĆČēĻĀĶ¼£ŗ
£Ø¢ņ£©ŅŅĶ¬Ń§Éč¼ĘĮĖĮķŅ»Ģ׏µŃé×°ÖĆČēĻĀĶ¼£ŗ
£Ø5£©ŹµŃéÖŠ“ż×¶ŠĪĘæÖŠ²»ŌŁ²śÉśĘųĢåŗ󣬓ņæŖ»īČūP“Óµ¼¹Ü×ó¶Ė»ŗ»ŗ¹ÄČėŅ»¶ØĮæµÄæÕĘų£¬ÕāŃł×öµÄÄæµÄŹĒ ”£
£Ø6£©³żŅŃ³ĘĮæµÄa g Na2SO3ѳʷĶā£¬ŹµŃéÖŠ»¹Ó¦²ā¶ØµÄŹż¾ŻŹĒ “¦£¬£ØĢīĶ¼ÖŠ×ÖÄø£©×°ÖĆŹµŃéĒ°ŗóµÄÖŹĮæ²ī”£
£Ø¢ó£©ĻÖÓŠŅŌĻĀŹŌ¼Į£ŗÕōĮóĖ®”¢ŃĪĖį”¢Ļ”ĻõĖį”¢BaCl2ČÜŅŗ”¢Ba£ØNO3£©2ČÜŅŗ”£
±ūĶ¬Ń§Óū“ÓÖŠŃ”ŌńŗĻŹŹŹŌ¼Į£¬Ą“²ā¶ØŅŃÖŖÖŹĮæĪŖa gµÄNa2SO3ѳʷ֊Na2SO3µÄÖŹ
Įæ·ÖŹż”£ĻĀŹöŹµŃé·½°øĄķĀŪÉĻæÉŠŠµÄÓŠ ”£
A£®½«ŃłĘ·Čܽā£¬¼Ó×ćĮæŃĪĖį£¬ŌŁ¼Ó×ćĮæBaCl2ČÜŅŗ£¬¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ”¢³ĘĮæµĆ³Įµķml g
B£®½«ŃłĘ·Čܽā£¬¼Ó×ćĮæĻõĖį£¬ŌŁ¼Ó×ćĮæBaCl2ČÜŅŗ”¢¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ”¢³ĘĮæµĆ³Įµķm2 g
C£®½«ŃłĘ·Čܽā£¬¼Ó¹żĮæBaCl2ČÜŅŗ£¬¹żĀĖŗó½«ČÜŅŗÕōøÉ£¬³ĘĮæµĆ¹ĢĢåm3g
D£®½«ŃłĘ·Čܽā£¬¼Ó¹żĮæBa£ØNO3£©2ČÜŅŗ£¬¹żĀĖ”¢Ļ“µÓ£¬ŌŚ³ĮµķÖŠ¼ÓČė×ćĮæŃĪĖį£¬ŌŁ¹żĀĖ£¬Ļ“µÓ”¢øÉŌļ£¬³ĘĮæµĆ¹ĢĢåm4g”£
”¾“š°ø”æ£Ø¢ń£©£Ø1£©¹Ų±ÕµÆ»É¼Š£Ø»ņÖ¹Ė®¼Š£©a£¬Óɳ¤¾±Ā©¶·Ļņ׶ŠĪĘæÄŚ¼ÓĖ®ÖĮĀ©¶·ÄŚŅŗĆęøßӌ׶ŠĪĘæÄŚŅŗĆę£¬¾²ÖĆŅ»»į¹Ū²ģŅŗĆęŹĒ·ń±ä»Æ£¬Čō²»±ä£¬ĖµĆ÷ĘųĆÜŠŌĮ¼ŗĆ£¬·ńŌņ”¢ĖµĆ÷×°ÖĆĀ©Ęų
£Ø2£©SO2+C12+2H2O === ![]() +2
+2![]() +4H
+4H![]()
MnO2+4HCl£ØÅØ£©![]() MnCl2+C12”ü+2H2O
MnCl2+C12ӟ+2H2O
£Ø3£©![]() ”Į100£„
”Į100£„
£Ø4£©×¶ŠĪĘæ¼°µ¼¹ÜÖŠµÄSO2Ī“Č«²æÅÅČėµ½¹ćæŚĘæÖŠÓėCl2ĶźČ«·“Ó¦»ņÓÉÓŚ²śÉśSO2ĖŁ¶Č½Ļæģ£¬Ī“ÓėCl2·“Ó¦¶ųÅÅČėµ½NaOHČÜŅŗÖŠ
£Ø¢ņ£©£Ø5£©½«×°ÖĆÖŠµÄSO2Č«²æÅÅČėµ½UŠĪ¹ÜÖŠ±»ĪüŹÕ
£Ø6£©D
£Ø¢ó£©£Ø7£©AD


 ½ņĒŽĢÓżŹī¼Ł°ĪøßĻĪ½Ó¹ć¶«ČĖĆń³ö°ęÉēĻµĮŠ“š°ø
½ņĒŽĢÓżŹī¼Ł°ĪøßĻĪ½Ó¹ć¶«ČĖĆń³ö°ęÉēĻµĮŠ“š°ø ²Ø²ØŠÜŹī¼Ł×÷Ņµ½Ī÷ČĖĆń³ö°ęÉēĻµĮŠ“š°ø
²Ø²ØŠÜŹī¼Ł×÷Ņµ½Ī÷ČĖĆń³ö°ęÉēĻµĮŠ“š°ø ѧ¶ųÓÅŹīĘŚĻĪ½ÓÄĻ¾©“óѧ³ö°ęÉēĻµĮŠ“š°ø
ѧ¶ųÓÅŹīĘŚĻĪ½ÓÄĻ¾©“óѧ³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
£Ø19·Ö£©øßĆĢĖį¼ŲŹĒĆĢµÄÖŲŅŖ»ÆŗĻĪļŗĶ³£ÓƵÄŃõ»Æ¼Į”£ŅŌĻĀŹĒŹµŃéŹŅÖŠÄ£Äā¹¤ŅµÉĻÓĆČķĆĢæóÖʱøøßĆĢĖį¼ŲµÄĮ÷³ĢĶ¼”£
£Ø1£©²Ł×÷¢ńµÄĆū³ĘĪŖ £»²Ł×÷¢óµÄĆū³ĘĪŖ ”£
£Ø2£©·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½ĪŖ ”£¹¤ŅµÉĻÓĆÉĻŹöŌĄķÉś²śKMnO4·½·Ø²śĀŹ½ĻµĶ£¬½ĻŗƵÄÖʱø·½·ØŹĒµē½ā·Ø”£ÓĆPt×÷Ńō¼«£¬Fe×÷Ņõ¼«£¬K2MnO4ĪŖµē½āŅŗ£¬Ńō¼«µÄµē¼«·“Ó¦Ź½ĪŖ ”£
£Ø3£©KMnO4ŹĒŅ»ÖÖ½ĻĪČ¶ØµÄ»ÆŗĻĪļ£¬µ«ČÕ¹ā¶ŌKMnO4ČÜŅŗµÄ·Ö½āÓŠ“ß»Æ×÷ÓĆ£¬Éś³ÉMnO2”¢KOHŗĶO2”£¶ųMnO2Ņ²ŹĒøĆ·Ö½ā·“Ó¦µÄŅ»Ö֓߻ƼĮ£¬ĒėÄćÉč¼ĘŅ»øöŹµŃé·½°ø£¬ŃéÖ¤MnO2¶ŌøĆ·Ö½ā·“Ó¦¾ßÓŠ“߻ƊŌ£ŗ ”£
£Ø4£©KMnO4ŌŚĖįŠŌ½éÖŹÖŠµÄĒæŃõ»ÆŠŌ¹ć·ŗÓ¦ÓĆÓŚ·ÖĪö»Æѧ֊”£
ĄżČē£ŗ2KMnO4+3H2SO4+5Na2SO35Na2SO4+K2SO4+2MnSO4+3H2O”£Ä³Ķ¬Ń§ÓĆKMnO4²ā¶ØŹµŃéŹŅ³¤ĘŚ“ę·ÅµÄNa2SO3¹ĢĢåµÄ“æ¶Č”£ĻÖÓū×¼Č·³ĘČ”6.3 gNa2SO3¹ĢĢåѳʷ£¬Åä³É500 mLČÜŅŗ”£Č”25.00 mLÉĻŹöČÜŅŗ·ÅČė׶ŠĪĘæÖŠ£¬ÓĆ0.01000 mol/L µÄĖįŠŌKMnO4ČÜŅŗ½ųŠŠµĪ¶Ø”£µĪ¶Ø½į¹ūČēĻĀ±ķĖłŹ¾£ŗ
| µĪ¶Ø“ĪŹż | “ż²āČÜŅŗµÄĢå»ż/mL | ±ź×¼ČÜŅŗµÄĢå»ż | |
| µĪ¶ØĒ°æĢ¶Č/mL | µĪ¶ØŗóæĢ¶Č/mL | ||
| 1 | 25.00 mL | 0.02 | 24.01 |
| 2 | 25.00 mL | 0.70 | 24.71 |
| 3 | 25.00 mL | 0.20 | 24.20 |
¢ŁÅäÖĘ500 mLNa2SO3ČÜŅŗŹ±£¬±ŲŠėÓƵ½µÄŹµŃéŅĒĘ÷ÓŠ£ŗÉÕ±”¢²£Į§°ō”¢½ŗĶ·µĪ¹Ü”¢Ņ©³×ŗĶ ”¢ ”£
¢ŚÅŠ¶ĻµĪ¶ØÖÕµćµÄŅĄ¾ŻŹĒ ”£
¢ŪĻĀĮŠ²Ł×÷»įµ¼ÖĀ²ā¶Ø½į¹ūĘ«øߵďĒ
A£®Ī“ÓƱź×¼ÅØ¶ČµÄĖįŠŌKMnO4ČÜŅŗČóĻ“µĪ¶Ø¹Ü
B£®µĪ¶ØĒ°×¶ŠĪĘæĪ“øÉŌļ
C£®µĪ¶ØĒ°µĪ¶Ø¹Ü¼ā×ģ²æ·ÖÓŠĘųÅŻ
D£®¹Ū²ģ¶ĮŹżŹ±£¬µĪ¶ØĒ°ŃöŹÓ£¬µĪ¶Øŗóø©ŹÓ
¢ÜÓĆÉĻŹöŹµŃ鏿¾Ż£¬¼ĘĖćNa2SO3µÄ“æ¶ČĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013-2014ѧğ¹ć¶«Ź”»ŻÖŻŹŠøßČżµŚČż“Īµ÷ŃŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŹµŃéĢā
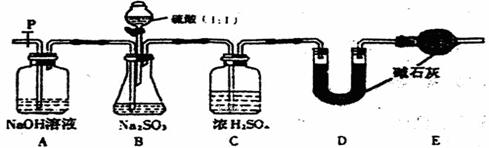
Na2SO3ŌŚæÕĘųÖŠŅ×±»Ńõ»Æ¶ų±äÖŹ”£Ä³Ķ¬Ń§ĪŖÖ¤Ć÷Na2SO3ÓŠ»¹ŌŠŌ£¬“ÓŅ»ĘæŹµŃéŹŅ³¤ĘŚ“ę·ÅµÄNa2SO3¹ĢĢåÖŠČ”³öÉŁĮæČÜÓŚĖ®£¬µĪČėŅ»¶ØĮæµÄÉÕ¼īČÜŅŗŗĶÉŁŠķäåĖ®£¬Õńµ“ŗóČÜŅŗ±äĪŖĪŽÉ«”£
£Ø1£©ŌŚ¼īŠŌČÜŅŗÖŠBr2ŗĶNa2SO3·“Ó¦µÄĄė×Ó·½³ĢŹ½ ”£
£Ø2£©·“Ó¦ŗóµÄČÜŅŗŗ¬ÓŠSO32-”¢SO42-”¢Br-”¢OH-µČŅõĄė×Ó£¬ĻĀ±ķŹĒijĶ¬Ń§¼ų¶ØĘäÖŠSO32-”¢SO42-ŗĶBr-µÄŹµŃé±Øøę£¬ĒėĶź³ÉĪ“ĢīĶźµÄ²æ·Ö”£
ĻŽŃ”ŹŌ¼Į£ŗ2 mol”¤L-1HCl£»1 mol”¤L-1 H2SO4£»l mol”¤L-1BaCl2£»l mol”¤L-1Ba(NO3)2£»1 mol”¤L-1 KMnO4”¢CCl4£»ŠĀÖʱ„ŗĶĀČĖ®£»Ę·ŗģČÜŅŗ”£
|
±ąŗÅ |
ŹµŃé²Ł×÷ |
Ō¤ĘŚĻÖĻóŗĶ½įĀŪ |
|
²½Öč¢Ł
|
ȔɣĮæ“ż²āŅŗ¼ÓČėŹŌ¹ÜÖŠ£¬¼ÓČė¹żĮæ2mol”¤L-1HCl£¬ŌŁµĪ¼ÓŹŹĮæ1 mol”¤L-1BaCl2 ČÜŅŗ”£ |
ÓŠ°×É«³ĮµķÉś³É£¬Ö¤Ć÷“ż²āŅŗÖŠŗ¬ÓŠ”¢SO42- ”£ |
|
²½Öč¢Ś
|
|
|
|
²½Öč¢Ū
|
|
|
£Ø3£©ĪŖĮĖ²ā¶ØÉĻŹöѳʷµÄ“æ¶Č£¬ĻÖČ”10.0æĖŹŌŃłÅä³É250mlČÜŅŗ”£Č”³ö25.00mlĖłÅäČÜŅŗ£¬ÓĆ0.10mol/LµÄĖįŠŌKMnO4ČÜŅŗµĪ¶ØÖĮÖÕµć”£·“Ó¦Ąė×Ó·½³ĢŹ½ĪŖ£ŗ
ÖŲø“²Ł×÷Čż“Ī£¬Ćæ“ĪĻūŗÄ0.10mol/L KMnO4ČÜŅŗĢå»ż·Ö±šĪŖ20.02 ml”¢ 20.00 mlŗĶ19.98 ml”££ØĻą¶ŌŌ×ÓÖŹĮæNa 23 S 32 O 16)
¢Ł¼ĘĖćѳʷ֊Na2SO3µÄÖŹĮæ·ÖŹżĪŖ ”££Ø½į¹ū±£Įō3Ī»ÓŠŠ§Źż×Ö£©
¢Ś²Ł×÷Ź±£¬ČōĪ“ÓĆ0.10mol/LµÄĖįŠŌKMnO4ČÜŅŗČóĻ“µĪ¶Ø¹Ü£¬»įµ¼ÖĀ²ā¶Ø½į¹ū ”££ØĢī”°Ę«øß”±”¢”°Ę«µĶ”±»ņ”°Ć»ÓŠÓ°Ļģ”±£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010ÄźÕć½Ź”ø߶žµŚ¶žŃ§ĘŚĘŚÖŠæ¼ŹŌ£Ø1-3°ą£©»ÆѧŹŌĢā ĢāŠĶ£ŗĢīæÕĢā
£Ø19·Ö£©øßĆĢĖį¼ŲŹĒĆĢµÄÖŲŅŖ»ÆŗĻĪļŗĶ³£ÓƵÄŃõ»Æ¼Į”£ŅŌĻĀŹĒŹµŃéŹŅÖŠÄ£Äā¹¤ŅµÉĻÓĆČķĆĢæóÖʱøøßĆĢĖį¼ŲµÄĮ÷³ĢĶ¼”£
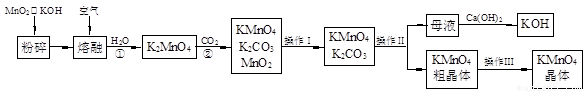
£Ø1£©²Ł×÷¢ńµÄĆū³ĘĪŖ £»²Ł×÷¢óµÄĆū³ĘĪŖ ”£
£Ø2£©·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½ĪŖ ”£¹¤ŅµÉĻÓĆÉĻŹöŌĄķÉś²śKMnO4·½·Ø²śĀŹ½ĻµĶ£¬½ĻŗƵÄÖʱø·½·ØŹĒµē½ā·Ø”£ÓĆPt×÷Ńō¼«£¬Fe×÷Ņõ¼«£¬K2MnO4ĪŖµē½āŅŗ£¬Ńō¼«µÄµē¼«·“Ó¦Ź½ĪŖ ”£
£Ø3£©KMnO4ŹĒŅ»ÖÖ½ĻĪČ¶ØµÄ»ÆŗĻĪļ£¬µ«ČÕ¹ā¶ŌKMnO4ČÜŅŗµÄ·Ö½āÓŠ“ß»Æ×÷ÓĆ£¬Éś³ÉMnO2”¢KOHŗĶO2”£¶ų MnO2Ņ²ŹĒøĆ·Ö½ā·“Ó¦µÄŅ»Ö֓߻ƼĮ£¬ĒėÄćÉč¼ĘŅ»øöŹµŃé·½°ø£¬ŃéÖ¤MnO2¶ŌøĆ·Ö½ā·“Ó¦¾ßÓŠ“߻ƊŌ£ŗ ”£
£Ø4£©KMnO4ŌŚĖįŠŌ½éÖŹÖŠµÄĒæŃõ»ÆŠŌ¹ć·ŗÓ¦ÓĆÓŚ·ÖĪö»Æѧ֊”£
ĄżČē£ŗ2KMnO4+3H2SO4+5Na2SO35Na2SO4+K2SO4+2MnSO4+3H2O”£Ä³Ķ¬Ń§ÓĆKMnO4²ā¶ØŹµŃéŹŅ³¤ĘŚ“ę·ÅµÄNa2SO3¹ĢĢåµÄ“æ¶Č”£ĻÖÓū×¼Č·³ĘČ”6.3 g Na2SO3¹ĢĢåѳʷ£¬Åä³É500 mLČÜŅŗ”£Č”25.00 mLÉĻŹöČÜŅŗ·ÅČė׶ŠĪĘæÖŠ£¬ÓĆ0.01000 mol/L µÄĖįŠŌKMnO4ČÜŅŗ½ųŠŠµĪ¶Ø”£µĪ¶Ø½į¹ūČēĻĀ±ķĖłŹ¾£ŗ
|
µĪ¶Ø“ĪŹż[Ą“Ō“:][Ą“Ō“:Z&xx&k.Com] |
“ż²āČÜŅŗµÄĢå»ż/mL[Ą“Ō“:ѧ#æĘ#ĶųZ#X#X#K] |
±ź×¼ČÜŅŗµÄĢå»ż[Ą“Ō“:] |
|
|
µĪ¶ØĒ°æĢ¶Č/mL |
µĪ¶ØŗóæĢ¶Č/mL |
||
|
1 |
25.00 mL |
0.02 |
24.01 |
|
2 |
25.00 mL |
0.70 |
24.71 |
|
3 |
25.00 mL |
0.20 |
24.20 |
¢ŁÅäÖĘ500 mLNa2SO3ČÜŅŗŹ±£¬±ŲŠėÓƵ½µÄŹµŃéŅĒĘ÷ÓŠ£ŗÉÕ±”¢²£Į§°ō”¢½ŗĶ·µĪ¹Ü”¢Ņ©³×ŗĶ ”¢ ”£
¢ŚÅŠ¶ĻµĪ¶ØÖÕµćµÄŅĄ¾ŻŹĒ ”£
¢ŪĻĀĮŠ²Ł×÷»įµ¼ÖĀ²ā¶Ø½į¹ūĘ«øߵďĒ
A£®Ī“ÓƱź×¼ÅØ¶ČµÄĖįŠŌKMnO4ČÜŅŗČóĻ“µĪ¶Ø¹Ü
B£®µĪ¶ØĒ°×¶ŠĪĘæĪ“øÉŌļ
C£®µĪ¶ØĒ°µĪ¶Ø¹Ü¼ā×ģ²æ·ÖÓŠĘųÅŻ
D£®¹Ū²ģ¶ĮŹżŹ±£¬µĪ¶ØĒ°ŃöŹÓ£¬µĪ¶Øŗóø©ŹÓ
¢ÜÓĆÉĻŹöŹµŃ鏿¾Ż£¬¼ĘĖćNa2SO3µÄ“æ¶ČĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
øßĆĢĖį¼ŲŹĒĆĢµÄÖŲŅŖ»ÆŗĻĪļŗĶ³£ÓƵÄŃõ»Æ¼Į”£ŅŌĻĀŹĒŹµŃéŹŅÖŠÄ£Äā¹¤ŅµÉĻÓĆČķĆĢæóÖʱøøßĆĢĖį¼ŲµÄĮ÷³ĢĶ¼”£
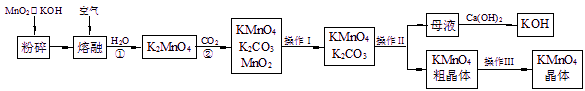
£Ø1£©²Ł×÷¢ńµÄĆū³ĘĪŖ £»²Ł×÷¢óµÄĆū³ĘĪŖ ”£
£Ø2£©·“Ó¦¢ŚµÄ»Æѧ·½³ĢŹ½ĪŖ ”£¹¤ŅµÉĻÓĆÉĻŹöŌĄķÉś²śKMnO4·½·Ø²śĀŹ½ĻµĶ£¬½ĻŗƵÄÖʱø·½·ØŹĒµē½ā·Ø”£ÓĆPt×÷Ńō¼«£¬Fe×÷Ņõ¼«£¬K2MnO4ĪŖµē½āŅŗ£¬Ńō¼«µÄµē¼«·“Ó¦Ź½ĪŖ ”£
£Ø3£©KMnO4ŹĒŅ»ÖÖ½ĻĪČ¶ØµÄ»ÆŗĻĪļ£¬µ«ČÕ¹ā¶ŌKMnO4ČÜŅŗµÄ·Ö½āÓŠ“ß»Æ×÷ÓĆ£¬Éś³ÉMnO2”¢KOHŗĶO2”£¶ų MnO2Ņ²ŹĒøĆ·Ö½ā·“Ó¦µÄŅ»Ö֓߻ƼĮ£¬ĒėÄćÉč¼ĘŅ»øöŹµŃé·½°ø£¬ŃéÖ¤MnO2¶ŌøĆ·Ö½ā·“Ó¦¾ßÓŠ“߻ƊŌ£ŗ ”£
£Ø4£©KMnO4ŌŚĖįŠŌ½éÖŹÖŠµÄĒæŃõ»ÆŠŌ¹ć·ŗÓ¦ÓĆÓŚ·ÖĪö»Æѧ֊”£
ĄżČē£ŗ2KMnO4+3H2SO4+5Na2SO3![]() 5Na2SO4+K2SO4+2MnSO4+3H2O”£Ä³Ķ¬Ń§ÓĆKMnO4²ā¶ØŹµŃéŹŅ³¤ĘŚ“ę·ÅµÄNa2SO3¹ĢĢåµÄ“æ¶Č”£ĻÖÓū×¼Č·³ĘČ”6.3 g Na2SO3¹ĢĢåѳʷ£¬Åä³É500 mLČÜŅŗ”£Č”25.00 mLÉĻŹöČÜŅŗ·ÅČė׶ŠĪĘæÖŠ£¬ÓĆ0.01000 mol/L µÄĖįŠŌKMnO4ČÜŅŗ½ųŠŠµĪ¶Ø”£µĪ¶Ø½į¹ūČēĻĀ±ķĖłŹ¾£ŗ
5Na2SO4+K2SO4+2MnSO4+3H2O”£Ä³Ķ¬Ń§ÓĆKMnO4²ā¶ØŹµŃéŹŅ³¤ĘŚ“ę·ÅµÄNa2SO3¹ĢĢåµÄ“æ¶Č”£ĻÖÓū×¼Č·³ĘČ”6.3 g Na2SO3¹ĢĢåѳʷ£¬Åä³É500 mLČÜŅŗ”£Č”25.00 mLÉĻŹöČÜŅŗ·ÅČė׶ŠĪĘæÖŠ£¬ÓĆ0.01000 mol/L µÄĖįŠŌKMnO4ČÜŅŗ½ųŠŠµĪ¶Ø”£µĪ¶Ø½į¹ūČēĻĀ±ķĖłŹ¾£ŗ
| µĪ¶Ø“ĪŹż | “ż²āČÜŅŗµÄĢå»ż/mL | ±ź×¼ČÜŅŗµÄĢå»ż | |
| µĪ¶ØĒ°æĢ¶Č/mL | µĪ¶ØŗóæĢ¶Č/mL | ||
| 1 | 25.00 mL | 0.02 | 24.01 |
| 2 | 25.00 mL | 0.70 | 24.71 |
| 3 | 25.00 mL | 0.20 | 24.20 |
¢ŁÅäÖĘ500 mLNa2SO3ČÜŅŗŹ±£¬±ŲŠėÓƵ½µÄŹµŃéŅĒĘ÷ÓŠ£ŗÉÕ±”¢²£Į§°ō”¢½ŗĶ·µĪ¹Ü”¢Ņ©³×ŗĶ ”¢ ”£
¢ŚÅŠ¶ĻµĪ¶ØÖÕµćµÄŅĄ¾ŻŹĒ ”£
¢ŪĻĀĮŠ²Ł×÷»įµ¼ÖĀ²ā¶Ø½į¹ūĘ«øߵďĒ
A£®Ī“ÓƱź×¼ÅØ¶ČµÄĖįŠŌKMnO4ČÜŅŗČóĻ“µĪ¶Ø¹Ü
B£®µĪ¶ØĒ°×¶ŠĪĘæĪ“øÉŌļ
C£®µĪ¶ØĒ°µĪ¶Ø¹Ü¼ā×ģ²æ·ÖÓŠĘųÅŻ
D£®¹Ū²ģ¶ĮŹżŹ±£¬µĪ¶ØĒ°ŃöŹÓ£¬µĪ¶Øŗóø©ŹÓ
¢ÜÓĆÉĻŹöŹµŃ鏿¾Ż£¬¼ĘĖćNa2SO3µÄ“æ¶ČĪŖ ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com