
| ||
| ||


 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��N2 ��O2 |
| B��CO��N2 |
| C��NO2 ��O3 |
| D��HCl��H2S |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Fe2O3�׳����죬��������ɫ�����Ϳ�� |
| B���������õİ뵼����� |
| C���������������������ά |
| D��ú̿�������Դ��ֱ��ȼ�ղ�����ɻ�����Ⱦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ˮ�� | B���մ� | C����ʯ�� | D���ռ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 175�� |
| �¶�/�� | 25 | 300 | 350 | 400 | 500 | 600 | 900 |
| ��������/g | 1.000 | 0.800 | 0.800 | 0.400 | 0.444 | 0.444 | 0.429 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��� | �� | �� | �� | �� |
| װ�� |  |  |  |  |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
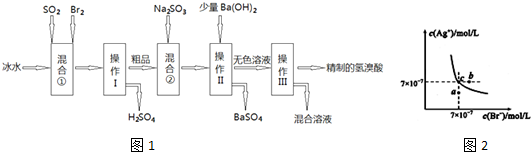
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��34Sԭ�Ӻ���������Ϊ16 |
| B��1H216O��1H218O����Է����������2 |
| C��13C��15Nԭ�ӵ�ԭ���������2 |
| D��2H+���OH-��������1H+�ĸ�ǿ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com