
���ڽ������ӵ�ij�ֺ�������ҩƤ�ɴ���ʯ��ˮ�ࡢ�衢�����������ƶ��ɡ��Իش�
��1��30Si��ԭ�ӵ�������Ϊ________��
��2��Al3����Yn���ĵ�������ͬ��Y������ĸ�Ԫ�ص��⻯���ˮ��Һ�������ԣ���n=________��
��3�����ӹ����У�ҩƤ�ڸ����²�����������ʹ�����������������壬��������________��
��4���������������36.0 g(����Fe2O3��Al2O3��SiO2)����������ϡ���ᣬ����õ�11.0 g���壻��Һ�м������NaOH��Һ������õ�21.4 g���壻���������Al2O3����������Ϊ________����ֻд�����
(1) 16����1�֣� (2)n=1 ��1�֣�
��3����CO2��2�֣� (4)25% ��3�֣�
��������
�����������1����ԭ�ӷ��ϵı���ʽ�����ϽDZ�ʾ�����������½DZ�ʾ��������������������������+�������ɼ����30Si��ԭ�ӵ�������Ϊ��30��14��16��
��2����Ԫ�����ڱ���ֻ�е� ��A��±��ԭ�ӵ��⻯���ˮ��Һ�ž������ԣ���ΪAl3����Yn���ĵ�������ͬ������Y��FԪ�ء�����n=1��
��3����ҩƤ�ijɷִ���ʯ��ˮ�ࡢ�衢�������ȿ�֪���ڸ�����ֻ�д���ʯ�ŷֽ����CO2���������ֻ����CO2���塣
��4��������ֻ��SiO2�������Ӧ�����11.0g��SiO2��������Fe2O3��Al2O3��������ֱ�����FeCl3��AlCl3������Һ�м������NaOH��ҺʱAlCl3����NaAlO2��FeCl3����Fe(OH)3����������21.4g������Fe(OH)3�������������ʵ���Ϊ ������ԭ���غ�֪Fe2O3�����ʵ���Ϊ0.1mol��������Ϊ0.1mol��160g?mol��1��16.0g��������Al2O3����������Ϊ
������ԭ���غ�֪Fe2O3�����ʵ���Ϊ0.1mol��������Ϊ0.1mol��160g?mol��1��16.0g��������Al2O3����������Ϊ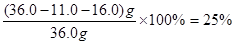 ��
��
���㣺�������ļ��㣻Ԫ�����ڱ�����ѧ����
���������⿼��Ԫ�����ڱ����Ų����ɺͻ�ѧ���㣬�ۺ��Խ�ǿ����Ŀ�ѶȽϴ�


 �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ģ�⿼�Ի�ѧ���⣨���� ���ͣ������
��9�֣����ڽ������ӵ�ij�ֺ�������ҩƤ�ɴ���ʯ��ˮ�ࡢ���������ƶ��ɡ�
��1��Al��ԭ�ӽṹʾ��ͼΪ________��Al��NaOH��Һ��Ӧ�����ӷ���ʽΪ_________��
(2)30Si��ԭ�ӵ�������Ϊ_________��SiO2����_________�������ӻۣ������
��3��Al3+��Yn���ĵ�������ͬ��Y������ĸ�Ԫ�ص��⻯���ˮ��Һ�������ԣ�������⻯���зе���͵���________��
��4�����ӹ����У�ҩƤ�ڸ����²�����������ʹ�����������������壬��������______��
��5���������������36.0g������Fe2O3��Al2O3��SiO2������������ϡ���ᣬ����õ�11.0g���壻��Һ�м������NaOH��Һ������õ�21.4g���壻���������Al2O3����������Ϊ__________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com