
��ʾ���б仯��ʽ����ȷ���ǣ� ��
A��NaHCO3ˮ������ӷ���ʽ��HCO![]() +H2O
+H2O ![]() H3O++CO
H3O++CO![]()
B��ʯ��ʯ���ڴ�������ӷ���ʽ��CaCO3+2H+=Ca2++CO2��+H2O
C�������绯ѧ��ʴ�ĸ�����Ӧ��4OH����4e��=2H2O+O2��
D��1 L 0.5mol/L H2SO4��Һ��1 L 1.0mol/L NaOH��Һ��Ӧ���ų�57.3kJ��������
1/2H2SO4(aq)+NaOH(aq)=1/2Na2SO4(aq)+H2O(l)����H=��57.3kJ/mol

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣���һ���¶��£���һ2L�̶��ݻ����ܱ�������ͨ��20 molN2��60 molH2������5���Ӻ�N2��Ũ����5mol�ML���ֹ���5���� N2 ��H2��NH3��Ũ�Ȳ��ٱ仯����ʱNH3��Ũ����14 mol�ML���ﵽƽ��ų�QKJ��������������������⣺
��ǰ5������H2��ʾ�Ļ�ѧ��Ӧ���ʣ� ����
�Ʒ�Ӧ�ﵽƽ���N2��ת���ʣ� ����
�DZ�ʾ���¶��ºϳɰ��Ļ�ѧƽ�ⳣ���ı���ʽΪ�� ����
����ƽ���������ѹǿ����ѧƽ���� �������ƶ�����������桱����������
�ɸ��¶��·�Ӧ���Ȼ�ѧ����ʽΪ�� �����ú�Q��ʽ�ӱ�ʾ����
���ڸ��¶��£�����һ2L�̶��ݻ����ܱ�������ͨ��N2 5 mol��H215 mol��NH330 mol����Ӧ�ﵽƽ���H2��Ũ���ǣ� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������ʡ�����еڶ�ʮ��ѧ�����߿��캽���ԣ�������ѧ�Ծ����������� ���ͣ������
����β�������е�һ����Ӧ���£�2NO(g)+2CO(g) N2(g)+2CO2(g)
N2(g)+2CO2(g)
��ش��������⣺
(1)��֪��N2(g)+O2(g)=2NO(g)����H= +180.5kJ/mol
C(s)+O2(g) =CO2(g)����H=" -393.5" kJ/mol
2C(s)+O2(g)=2CO(g)����H= -221kJ/mol
��2NO(g)+2CO(g) N2(g) +2CO2(g)�ġ�H=___________��
N2(g) +2CO2(g)�ġ�H=___________��
(2)��һ���¶��£���һ���ΪV�����ܱ������г���һ������NO��CO2��t1ʱ�̴ﵽƽ��״̬����ʱn(CO)="a" mol��n(NO)="2a" mol��n(N2)="b" mol����N2ռƽ���������1/4��
����÷�Ӧ��ƽ�ⳣ��K=_______ ____��(��ֻ��a��V��ʽ�ӱ�ʾ)
���жϸ÷�Ӧ�ﵽƽ��ı�־��____ _____
A��v����(CO2)=v����(CO) B�����������ܶȲ��ٸı�
C����������ƽ����Է����������ٸı� D��NO��CO��N2��CO2��Ũ�Ⱦ����ٱ仯
����t2ʱ�̣����������ݻ�Ѹ������ԭ����2�����������������������£�t3ʱ�̴ﵽ�µ�ƽ��״̬��������ͼ�в��仭����t2��t4ʱ������Ӧ������ʱ��ı仯���ߣ�
(3)���Ҫ��������β��ͬʱ��߸÷�Ӧ�����ʺ�NO��ת���ʣ���ȡ�Ĵ�ʩ��___________
A�������¶� B������ѹǿͬʱ�Ӵ���
C�������¶�ͬʱ����N2 D����ʱ��CO2��N2�ӷ�Ӧ��ϵ������
(4)Ϊ��������β���е��к�����Դ�������Ⱦ����������װβ������װ�á�����װ����װ�к�Pd�ȹ���Ԫ�صĴ����������ڴ�������������������õĻ�������ͼ��ʾ��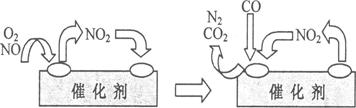
��д���˱仯�е��ܻ�ѧ��Ӧ����ʽ��________________________________________��
�����������������Ʒ�Ӧ2CO(g)=2C(s)+O2(g)������CO����Ⱦ�������ж��Ƿ���в�˵�����ɣ�_____________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����������к�ԭһ�и߶��ڶ�ѧ�ο��Ի�ѧ�Ծ� ���ͣ������
��12�֣���һ���¶��£���һ2L�̶��ݻ����ܱ�������ͨ��20 molN2��60 molH2������5���Ӻ�N2��Ũ����5mol�ML���ֹ���5���� N2��H2��NH3��Ũ�Ȳ��ٱ仯����ʱNH3��Ũ����14 mol�ML���ﵽƽ��ų�QKJ��������������������⣺
��ǰ5������H2��ʾ�Ļ�ѧ��Ӧ���ʣ� ����
�Ʒ�Ӧ�ﵽƽ���N2��ת���ʣ� ����
�DZ�ʾ���¶��ºϳɰ��Ļ�ѧƽ�ⳣ���ı���ʽΪ�� ����
����ƽ���������ѹǿ����ѧƽ���� �������ƶ�����������桱����������
�ɸ��¶��·�Ӧ���Ȼ�ѧ����ʽΪ�� �����ú�Q��ʽ�ӱ�ʾ����
���ڸ��¶��£�����һ2L�̶��ݻ����ܱ�������ͨ��N2 5 mol��H215 mol��NH330 mol����Ӧ�ﵽƽ���H2��Ũ���ǣ� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ������������ظ�����ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
�����꣬������ȾԽ��Խ���أ��������������ǵ�������С����彡�������������Ӱ�졣����β������Ҫ�Ĵ�����ȾԴ����������β��Σ���ķ���֮һ�����������ϰ�װ��ת�����������ķ�ӦΪ��
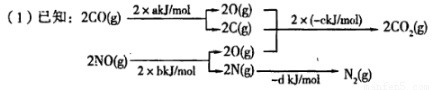
�� ,��H=__________kJ
,��H=__________kJ ���ú�a��b��c��d��ʽ�ӱ�ʾ����
���ú�a��b��c��d��ʽ�ӱ�ʾ����
��2��ij�¶��£����ݻ�Ϊ1L�������г���3 mol NO��1 mol CO, NO��ת������ʱ��ı仯����ͼ��ʾ��
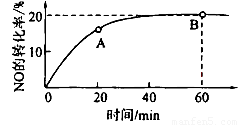
�ش��������⣺
�����¶��£���ѧƽ�ⳣ��K=___________��ƽ��ʱCO��ת����Ϊ__________��
��A����淴Ӧ������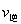 (CO)___________B����淴Ӧ����
(CO)___________B����淴Ӧ����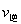 (NO)������>������<������=������
(NO)������>������<������=������
������ͼ����ȷ���ܱ�����ʱ��T1ʱ�̷�Ӧһ������ƽ��״̬����__________��
����÷�Ӧ��ƽ������¶ȣ�ƽ�ⳣ����������H___________0������>������< ������=����
����ƽ����������ݻ�����һ��������˵����ȷ����
A��ƽ��������Ӧ�����ƶ�? B��CO�������������
C��ƽ�ⳣ����С????????? D��һ����̼��Ũ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ�����и����߿��캽���ԣ�������ѧ�Ծ��������棩 ���ͣ������
����β�������е�һ����Ӧ���£�2NO(g)+2CO(g) N2(g)+2CO2(g)
N2(g)+2CO2(g)
��ش��������⣺
(1)��֪��N2(g)+O2(g)=2NO(g)����H= +180.5kJ/mol
C(s)+O2(g) =CO2(g)����H=" -393.5" kJ/mol
2C(s)+O2(g)=2CO(g)����H= -221kJ/mol
��2NO(g)+2CO(g) N2(g) +2CO2(g)�ġ�H=___________��
N2(g) +2CO2(g)�ġ�H=___________��
(2)��һ���¶��£���һ���ΪV�����ܱ������г���һ������NO��CO2��t1ʱ�̴ﵽƽ��״̬����ʱn(CO)="a" mol��n(NO)="2a" mol��n(N2)="b" mol����N2ռƽ���������1/4��
����÷�Ӧ��ƽ�ⳣ��K=_______ ____��(��ֻ��a��V��ʽ�ӱ�ʾ)
���жϸ÷�Ӧ�ﵽƽ��ı�־��____ _____
A��v����(CO2)=v����(CO) B�����������ܶȲ��ٸı�
C����������ƽ����Է����������ٸı� D��NO��CO��N2��CO2��Ũ�Ⱦ����ٱ仯
����t2ʱ�̣����������ݻ�Ѹ������ԭ����2�����������������������£�t3ʱ�̴ﵽ�µ�ƽ��״̬��������ͼ�в��仭����t2��t4ʱ������Ӧ������ʱ��ı仯���ߣ�

(3)���Ҫ��������β��ͬʱ��߸÷�Ӧ�����ʺ�NO��ת���ʣ���ȡ�Ĵ�ʩ��___________
A�������¶� B������ѹǿͬʱ�Ӵ���
C�������¶�ͬʱ����N2 D����ʱ��CO2��N2�ӷ�Ӧ��ϵ������
(4)Ϊ��������β���е��к�����Դ�������Ⱦ����������װβ������װ�á�����װ����װ�к�Pd�ȹ���Ԫ�صĴ����������ڴ�������������������õĻ�������ͼ��ʾ��
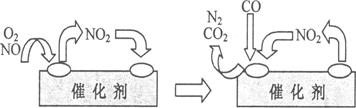
��д���˱仯�е��ܻ�ѧ��Ӧ����ʽ��________________________________________��
�����������������Ʒ�Ӧ2CO(g)=2C(s)+O2(g)������CO����Ⱦ�������ж��Ƿ���в�˵�����ɣ�_____________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com