
£Ø5·Ö£©ĻÖÓŠŅ»ĘæÅضČĪŖ0.2 mol/LµÄijĖįČÜŅŗ£¬æÉÄÜĪŖ“×Ėį”¢ŃĪĖį”¢ĮņĖįÖŠµÄŅ»ÖÖ”£ĪŖĮĖČ·¶ØøĆĖįČÜŅŗµÄ×é³É½ųŠŠŹµŃé£ŗČ”25.00 mL0.1 mol/LµÄĒāŃõ»ÆÄĘČÜŅŗ£¬ÖšµĪ¼ÓČėøĆĖįČÜŅŗ£¬Ē”ŗĆ·“Ó¦ĶźČ«Ź±ĖłŠčøĆĖįČÜŅŗĢå»żĪŖ12.50 mL”£Ēė»Ų“š£ŗ
£Ø1£©øĆĖį²»æÉÄÜŹĒ £»
£Ø2£©ÓĆpHŹŌÖ½²āµĆ·“Ó¦ŗóĖłµĆČÜŅŗ³Ź¼īŠŌ£¬øł¾Ż“ĖĻÖĻóĖµĆ÷øĆĖįČÜŅŗĪŖ £¬ÓĆĄė×Ó·½³ĢŹ½ĖµĆ÷ČÜŅŗ³Ź¼īŠŌµÄŌŅņ £»
£Ø3£©ŹµŃéÖŠµĪ¶ØĒśĻßČēÓŅĶ¼£¬ŌŚBµć£¬a 12.5£ØĢī“óÓŚ”¢Š”ÓŚ»ņµČÓŚ£©ŌŚCµćø÷Ąė×ÓÅضČÓɓ󵽊”ÅÅŠņ ”£

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010Äź±±¾©ŹŠ°ĖŅ»ÖŠŃ§ø߶žµŚ¶žŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø5·Ö£©ĻÖÓŠŅ»ĘæÅضČĪŖ0.2 mol/LµÄijĖįČÜŅŗ£¬æÉÄÜĪŖ“×Ėį”¢ŃĪĖį”¢ĮņĖįÖŠµÄŅ»ÖÖ”£ĪŖĮĖČ·¶ØøĆĖįČÜŅŗµÄ×é³É½ųŠŠŹµŃé£ŗČ”25.00 mL0.1 mol/LµÄĒāŃõ»ÆÄĘČÜŅŗ£¬ÖšµĪ¼ÓČėøĆĖįČÜŅŗ£¬Ē”ŗĆ·“Ó¦ĶźČ«Ź±ĖłŠčøĆĖįČÜŅŗĢå»żĪŖ12.50 mL”£Ēė»Ų“š£ŗ
£Ø1£©øĆĖį²»æÉÄÜŹĒ £»
£Ø2£©ÓĆpHŹŌÖ½²āµĆ·“Ó¦ŗóĖłµĆČÜŅŗ³Ź¼īŠŌ£¬øł¾Ż“ĖĻÖĻóĖµĆ÷øĆĖįČÜŅŗĪŖ £¬ÓĆĄė×Ó·½³ĢŹ½ĖµĆ÷ČÜŅŗ³Ź¼īŠŌµÄŌŅņ £»
£Ø3£©ŹµŃéÖŠµĪ¶ØĒśĻßČēÓŅĶ¼£¬ŌŚBµć£¬a 12.5£ØĢī“óÓŚ”¢Š”ÓŚ»ņµČÓŚ£©ŌŚCµćø÷Ąė×ÓÅضČÓɓ󵽊”ÅÅŠņ ”£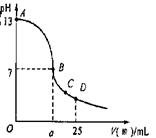
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010Äź±±¾©ŹŠø߶žµŚ¶žŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø5·Ö£©ĻÖÓŠŅ»ĘæÅضČĪŖ0.2 mol/LµÄijĖįČÜŅŗ£¬æÉÄÜĪŖ“×Ėį”¢ŃĪĖį”¢ĮņĖįÖŠµÄŅ»ÖÖ”£ĪŖĮĖČ·¶ØøĆĖįČÜŅŗµÄ×é³É½ųŠŠŹµŃé£ŗČ”25.00 mL0.1 mol/LµÄĒāŃõ»ÆÄĘČÜŅŗ£¬ÖšµĪ¼ÓČėøĆĖįČÜŅŗ£¬Ē”ŗĆ·“Ó¦ĶźČ«Ź±ĖłŠčøĆĖįČÜŅŗĢå»żĪŖ12.50 mL”£Ēė»Ų“š£ŗ
£Ø1£©øĆĖį²»æÉÄÜŹĒ £»
£Ø2£©ÓĆpHŹŌÖ½²āµĆ·“Ó¦ŗóĖłµĆČÜŅŗ³Ź¼īŠŌ£¬øł¾Ż“ĖĻÖĻóĖµĆ÷øĆĖįČÜŅŗĪŖ £¬ÓĆĄė×Ó·½³ĢŹ½ĖµĆ÷ČÜŅŗ³Ź¼īŠŌµÄŌŅņ £»
£Ø3£©ŹµŃéÖŠµĪ¶ØĒśĻßČēÓŅĶ¼£¬ŌŚBµć£¬a 12.5£ØĢī“óÓŚ”¢Š”ÓŚ»ņµČÓŚ£©ŌŚCµćø÷Ąė×ÓÅضČÓɓ󵽊”ÅÅŠņ ”£

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
(1)øĆĖį²»æÉÄÜŹĒ________________£»
(2)ÓĆpHŹŌÖ½²āµĆ·“Ó¦ŗóĖłµĆČÜŅŗ³Ź¼īŠŌ£¬øł¾Ż“ĖĻÖĻóĖµĆ÷øĆĖįČÜŅŗĪŖ__________________£¬ÓĆĄė×Ó·½³ĢŹ½ĖµĆ÷ČÜŅŗ³Ź¼īŠŌµÄŌŅņ_______________________________________________£»
(3)·“Ó¦ŗóĖłµĆČÜŅŗÖŠĄė×ÓÅضČÓɓ󵽊”µÄĖ³ŠņŹĒ____________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻÖÓŠŅ»ĘæÅضČĪŖ0.2 mol/LµÄijĖįČÜŅŗ£¬æÉÄÜĪŖ“×Ėį”¢ŃĪĖį”¢ĮņĖįÖŠµÄŅ»ÖÖ”£ĪŖĮĖČ·¶ØøĆĖįČÜŅŗµÄ×é³É½ųŠŠŹµŃé£ŗČ”25.00 mL0.1 mol/LµÄĒāŃõ»ÆÄĘČÜŅŗ£¬ÖšµĪ¼ÓČėøĆĖįČÜŅŗ£¬Ē”ŗĆ·“Ó¦ĶźČ«Ź±ĖłŠčøĆĖįČÜŅŗĢå»żĪŖ12.50 mL”£Ēė»Ų“š£ŗ
£Ø1£©øĆĖį²»æÉÄÜŹĒ £»
£Ø2£©ÓĆpHŹŌÖ½²āµĆ·“Ó¦ŗóĖłµĆČÜŅŗ³Ź¼īŠŌ£¬øł¾Ż“ĖĻÖĻóĖµĆ÷øĆĖįČÜŅŗĪŖ £¬ÓĆĄė×Ó·½³ĢŹ½ĖµĆ÷ČÜŅŗ³Ź¼īŠŌµÄŌŅņ £»
£Ø3£©ŹµŃéÖŠµĪ¶ØĒśĻßČēÓŅĶ¼£¬ŌŚBµć£¬a 12.5£ØĢī“óÓŚ”¢Š”ÓŚ»ņµČÓŚ£©ŌŚCµćø÷Ąė×ÓÅضČÓɓ󵽊”ÅÅŠņ ”£
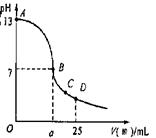
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com