


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
+ 4 |
2- 3 |
2- 4 |
| ʵ�� ��� |
ʵ������ | ʵ���� |
| 1 | ��AgNO3��Һ | �а�ɫ�������� |
| 2 | ������NaOH��Һ������ | �ռ�������1.12L��������ɱ�״���µ������ |
| 3 | ������BaCl2��Һʱ�������ó�������ϴ�ӡ������������������м�����ϡ���ᣬȻ�������� | ��һ�γ�������Ϊ6.27g���ڶ��γ�������Ϊ2.33g |
| �����ӷ��� | ���ʵ���Ũ�ȣ�mol/L�� | ||||
SO
SO
|
0.1 0.1 | ||||
CO
CO
|
0.2 0.2 | ||||
+ 4 |
2- 3 |
2- 4 |
+ 4 |
2- 3 |
2- 4 |
+ 4 |
2- 3 |
2- 4 |
+ 4 |
2- 3 |
2- 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ɼ������ӻ�������ɵĻ����������������е������֣�K+��![]() ��Mg2+��Ba2+��CI-��
��Mg2+��Ba2+��CI-�� ![]() ��
�� ![]() �����û��������ˮ��ó�����Һ����ȡ3��l00mL����Һ�ֱ��������ʵ�飺
�����û��������ˮ��ó�����Һ����ȡ3��l00mL����Һ�ֱ��������ʵ�飺
| ������� | �������� | ������ |
| ||
| 1 | ��AgNO3��Һ | �а�ɫ�������� |
| ||
| 2 | ������NaOH��Һ������ | �ռ�������1.12L��������� |
| ||
| ��״���µ������ |
| ||||
| 3 | ������BaCl2��Һʱ�������ó�������ϴ�ӡ������������������м�����ϡ���ᣬȻ �������� | ��һ�γ�������Ϊ6.27g,�� ���γ�������Ϊ2.33g |
| ||
�Իش��������⣺
��1������ʵ��1��Cl-�Ƿ���ڵ��ж��� (�һ�����ڡ�����һ�������ڡ�����ȷ����)������ʵ��l��3�жϻ������һ�������ڵ������� ��
��2����ȷ����Һ��һ�����ڵ������Ӽ������ʵ���Ũ��(�ɲ�����)��
| �����ӷ��� | ���ʵ���Ũ�ȣ�mo1��L-�� | |
��3����ȷ��K- �Ƿ����? ���жϵ�������
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ɹŰ�������һ�и���9���¿���ѧ�Ծ����������� ���ͣ������
��18�֣��ɼ������ӻ�������ɵĻ����������������е������֣�K����Cl����NH4+��Mg2����Ba2����CO32����SO42�������û��������ˮ��ó�����Һ����ȡ����100 mL����Һ�ֱ��������ʵ�飺
| ʵ����� | ʵ������ | ʵ���� |
| 1 | ��AgNO3��Һ | �а�ɫ�������� |
| 2 | ������NaOH��Һ������ | �ռ�������1.12 L(������ɱ�״���µ����) |
| 3 | ������BaCl2��Һ�������ó�������ϴ�ӡ������������������м�����ϡ���ᣬȻ�������� | ��һ�γ�������Ϊ6.27 g���ڶ��γ�������Ϊ2.33 g�� |
| �����ӷ��� | ���ʵ���Ũ��(mol��L��1) |
| | |
| | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��㶫ʡ���ڸ���ѧ������ѧ�ڵ�һ���¿���ѧ���� ���ͣ������
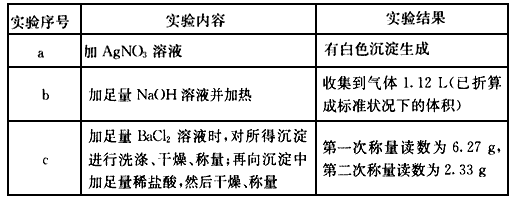
��10�֣��ɼ������ӻ�������ɵĻ����������������е������֣�K+��NH4+��Mg2+��Cu2+��Ba2+��C1����SO42����CO32-�����û��������ˮ�����ɫ������Һ���ֱַ�ȡ3��100mL����Һ��������ʵ�飺
| ʵ����� | ʵ������ | ʵ���� |
| a | ��AgNO3��Һ | �а�ɫ�������� |
| b | ������NaOH��Һ������ | �ռ�������1.12L��������ɱ� ״���µ������ |
| c | ������BaC12��Һʱ�������ó�������ϴ�ӡ������������������м�����ϡ���ᣬȻ�������� | ��һ�γ�������Ϊ6. 27g���ڶ��� ��������Ϊ2.33g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��³�ư���л�ѧѡ��6 3.1 ������ֵļ�����ϰ���������棩 ���ͣ������
�ɼ������ӻ�������ɵĻ����������������е������֣�K����Cl����NH4+��Mg2����CO32-��Ba2����SO42-�����û��������ˮ��ó�����Һ����ȡ3��100 mL����Һ�ֱ��������ʵ�飺
|
ʵ����� |
ʵ������ |
ʵ���� |
|
1 |
��AgNO3��Һ |
�а�ɫ�������� |
|
2 |
������NaOH��Һ������ |
�ռ�������1.12 L(������ɱ�״���µ����) |
|
3 |
������BaCl2��Һʱ�������ó�������ϴ�ӡ������������������м�����ϡ���ᣬȻ�������� |
��һ�γ�������Ϊ6.27 g���ڶ��γ�������Ϊ2.33 g |
�Իش��������⣺
(1)����ʵ��1��Cl���Ƿ���ڵ��ж���________(�һ�����ڡ�����һ�������ڡ�����ȷ����)������ʵ��1��3�жϻ������һ�������ڵ�������________��
(2)��ȷ����Һ��һ�����ڵ������Ӽ������ʵ���Ũ��(�ɲ�����)��
|
�����ӷ��� |
���ʵ���Ũ��/mol��L��1 |
|
|
|
|
|
|
(3)��ȷ��K���Ƿ���ڣ�________���жϵ�������
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com