
��1�����״����4.48LCO2��������ԭ����Ŀ��ͬ��ˮ�������� g��
��2��V L Fe2��S04��3��Һ�к�Fe3+m g������Һ��SO4 2-�����ʵ���Ũ��Ϊ mol��L��
2-�����ʵ���Ũ��Ϊ mol��L��
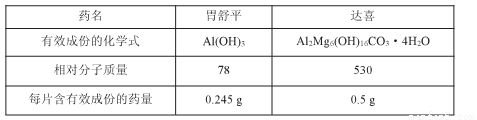
��3��9.2g���������NOx���к���ԭ��0.2mol����x����ֵΪ________________��
��4��0.4molij��������Ϊ9.8L����������Ħ�����Ϊ ����������������__________����ǡ����ǡ�����״����
��5�������dz��õ��к�θ���ҩ�
����10Ƭθ��ƽ��5Ƭ��ϲ�����������ʵ����϶���� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��㶫ʡ��ͷ�и߶���ѧҵˮƽ���Ի�ѧ���������棩 ���ͣ�ѡ����
������Ƽ��˵��������ǣ� ��
A����Ƽ��ǹ��ʰ�ί���ϸ��ֹʹ�õ��˷ܼ�
B����Ƽ��Ǵ���ҩ����ȡ����Ȼҩ��
C����Ƽ���ʹ���˷ܣ��˶�Ա���ú��ܳ�ˮƽ����
D����Ƽ������ߵ�Ч��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��㶫ʡ��ͷ�и߶���ѧҵˮƽ���Ի�ѧ���������棩 ���ͣ�ѡ����
��������ЧӦ��ԭ����Ҫ�Ǵ����к��д����ģ� ��
A��SO2 B��NO2 C��CO2 D��CO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��㶫ʡ��ͷ�и߶���ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
��һ���¶��£���a L�ܱ������м���1 mol X�����2 mol Y���壬�������·�Ӧ��X��g��+2Y��g��  3Z��g�����˷�Ӧ�ﵽƽ��ı�־�ǣ� ��
3Z��g�����˷�Ӧ�ﵽƽ��ı�־�ǣ� ��
A��������ѹǿ����ʱ��仯
B�������ڸ����ʵ�Ũ�Ȳ���ʱ��仯
C��������X��Y��Z��Ũ��֮��Ϊl:2:3
D����λʱ������0.1molXͬʱ����0.3molZ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��㶫ʡ��ͷ�и߶���ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵����ȷ���ǣ� ��
A����֪C2H6��ȼ����Ϊ1090 kJ��mol��1�����ʾC2H6ȼ���ȵ��Ȼ�ѧ����ʽΪ��
C2H6��g����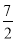 O2��g�� = 2CO2��g����3H2O��g�� ��H����1090 kJ��mol��1
O2��g�� = 2CO2��g����3H2O��g�� ��H����1090 kJ��mol��1
B����֪2CO��g����O2��g�� = 2CO2��g�� ��H����566 kJ��mol��1����CO��ȼ���Ȧ�H����283 kJ
C���ⶨHCl��NaOH��Ӧ���к���ʱ��ÿ��ʵ���Ӧ����3���¶�
D����ϡ��Һ�У�H+��aq��+OH-��aq��=H2O��l�� ��H=��57.3kJ��mol��1��˵��ϡ������ϡNaOH��Һ��Ӧ����1 mol H2O��l��ʱ�ų�57.3 kJ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�긣��ʡ������У��һ��ѧ������������ѧ�Ծ��������棩 ���ͣ�ѡ����
���й�����Һ���ʵ���Ũ�ȵ�˵����ȷ���ǣ� ��
A����״���£���22.4 L HC1�ܶ�1Lˮ�������1 mol��L-l��ϡ����
B����100 mL 18 mol.L-1��ŨH2SO4��100 mLˮ��ϣ������9 moI.L-l��H2SO4��Һ
18 mol.L-1��ŨH2SO4��100 mLˮ��ϣ������9 moI.L-l��H2SO4��Һ
C����4.0 g NaOH����100 mL����ƿ�У���ˮ���̶��ߣ����1 mol-L_1��NaOH��Һ
D����0.1 mol NaCI���100 mL��Һ������ȡ��10 mL����ȡ����Һ�����ʵ���Ũ��Ϊ1 mol��L-l
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�긣��ʡ������У��һ��ѧ������������ѧ�Ծ��������棩 ���ͣ�ѡ����
ij��������ֻ�����ƺ�������Ԫ�أ���������������ˮ�з�Ӧ�����������壬�����������ֿ�����һ�������·�Ӧţ��ˮ����ԭ�������ʵ�����ǣ� ��
A��Na2O2��Na2O B��Na2O2 C��Na2O2��Na D��Na��Na2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʦ���и߶�����������ѧ�Ծ��������棩 ���ͣ�ʵ����
���к͵ζ����ⶨij��������ʵ���Ũ�ȡ�
��1������ҺӦʢ��________���A����B�����ζ����С�
��2����ѡ�÷�̪��ָʾ������0.125 0 mol��L��1�ı�����������Һ�ζ�������жϵζ��յ�________________________��
��3��ʵ�����ݼ�¼���±�����������ݲ����㣬��������ʵ���Ũ�ȣ�__________mol��L��1��
�ζ����� | ������Һ���/mL | ||
����Һ | |||
�ζ�ǰ����/mL | �ζ������/mL | ||
1 | 20.00 | 0.00 | 16.02 |
2 | 20.00 | 0.00 | 15.98 |
3 | 20.00 | 0.00 | 16.00 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ��һ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����й�ʵ���������ȷ���ǣ� ��

A.��δ֪Һ����ζʱ��Ӧ�ý��Լ�ƿ�ھ���ڱ�ԶһЩ�����������ȶ�
B�����ⵥ�ʴӵ��CCl4��Һ�з������������ͼ����ʾ ʵ��װ��
ʵ��װ��
C��������ƿ���ƺ�һ��Ũ�ȵ�ij��Һ������ƿ����������ƿ�����ϱ�ǩ
D������500 mL 0.4 mol��L��1NaCl��Һ����Ҫ��������ͼ����ʾ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com