
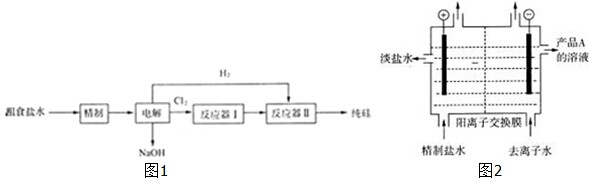
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||


 ���ɶ��ȫ���ƿؾ�ϵ�д�
���ɶ��ȫ���ƿؾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���������ʱ������������� |
| B����������ʱ�ܶ��п������ |
| C��������ʱ������������� |
| D��ԭ�������ʱ������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������NO-3�����ܺ�Fe3+ |
| B������NO3-��Cl-��I- |
| C����I-��������ȷ���Ƿ�Cl- |
| D������Fe3+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���������еμӰ�ˮ��H++OH-=H2O | ||||
B���Ȼ����������������Ũ��Һ��ϼ��ȣ�NH4++OH-
| ||||
| C��������þ��ϡ���ᷴӦ��H++OH-�TH2O | ||||
| D������ͭ��ϡ���ᷴӦ��Cu+2H++2NO3-�TCu2++2NO��+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
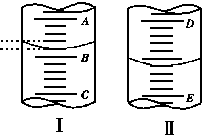 ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ�������ָʾ��������д���пհף�
ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ�������ָʾ��������д���пհף�| �ζ����� | ��������������Һ�����/mL | 0.1000mol��L-1 ��������/mL | ||
| �ζ�ǰ�̶� | �ζ���̶� | Һ�����/mL | ||
| ��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
| �ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
| ������ | 25.00 | 0.22 | 26.31 | 26.09 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 CH2COOC2H5+CH3CH2OH
CH2COOC2H5+CH3CH2OH ���ϳɻ����������·���£�
���ϳɻ����������·���£�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
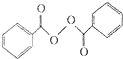 ����ȥ���������������������Ŀǰ�ѱ����ã��ϳɹ��������������������£�
����ȥ���������������������Ŀǰ�ѱ����ã��ϳɹ��������������������£�
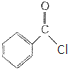 ������ˮ��Ӧ���ɱ����ᣬͬ��Ҳ�����Ҵ���Ӧ��д�������Ҵ���Ӧ�����л����������
������ˮ��Ӧ���ɱ����ᣬͬ��Ҳ�����Ҵ���Ӧ��д�������Ҵ���Ӧ�����л�����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��H2S |
| B��S |
| C��FeS |
| D��FeSO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
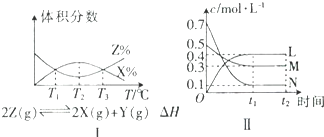
| A����ͼ���֪���÷�Ӧ��T2ʱ�ﵽƽ�� |
| B���ɢ�ͼ��֪���÷�Ӧ�ġ�H��0 |
| C��ͼ������Ӧ�Ļ�ѧ����ʽΪ2M+6N?3L |
| D����ͼ���֪�����ܱ�������M��N��L����ʼŨ������Ϊ0.4mol/L��0.4mol/L��0.2mol/L�����������������䣬�ﵽƽ��ʱL��Ũ��Ϊ0.4mol/L |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com