
�Իش��������⣺
��ָ������ʵ����Ʒ������(��ϴ�Ӹɾ�)ʹ��ʱ�ĵ�һ��������
��ʯ����ֽ(������������)
������ƿ  (2)����ʵ������ѡ�õ�����������Լ����ۺ�������____________��
(2)����ʵ������ѡ�õ�����������Լ����ۺ�������____________��
A����������ƽ����5.85 g�Ȼ��ƾ��� | B����������ˮ��pH��ֽ���Ϳ��Լ���pH��ȵ�H2SO4��Һ��CH3COOH��Һ | C���ü�ʽ�ζ�����ȡ25.00 mL���������Һ | D�������ô����������������ơ�̼���� |
 E. ��Ͳ��Һ���������Ϊ10.0 mLʱ��ȫ�������ձ��ڵ�ʵ�����Ҳ��10.0 mL
E. ��Ͳ��Һ���������Ϊ10.0 mLʱ��ȫ�������ձ��ڵ�ʵ�����Ҳ��10.0 mL F. 100 mL����ƿ��Һ�����ôﵽ�̶���ʱ��ȫ�������ձ��ڵ�ʵ�����Ҳ��Ϊ100 mL
F. 100 mL����ƿ��Һ�����ôﵽ�̶���ʱ��ȫ�������ձ��ڵ�ʵ�����Ҳ��Ϊ100 mL (3)����ȡ15.00 mL Na2CO3��Һ��Ӧѡ�õ�������_________________��
(3)����ȡ15.00 mL Na2CO3��Һ��Ӧѡ�õ�������_________________�� (4)������ͭ������ᾧˮ�����IJⶨʵ���У�������������Ҫ����___________�Ρ�
(4)������ͭ������ᾧˮ�����IJⶨʵ���У�������������Ҫ����___________�Ρ�  ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ������ | SO42-��NO3-��Cl- |
| ������ | Fe3+��Fe2+��NH4+��Cu2+��Al3+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ������ | SO42-��NO3-��Cl- |
| ������ | Fe3+��Fe2+��NH4+��Cu2+��Al3+ |
| 1 |
| 22.4 |
| 1 |
| 22.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
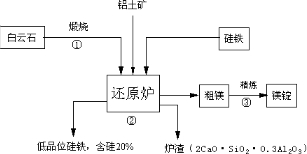
�������ط�ұ��þ�Ĺ�����������ͼ��¯���г������հ���ʯ�����⣬���������չ���������Al2O3����ҪĿ���ǽ��������۵㣬����Һ̬������
��֪����ʯ�ijɷ�ΪCaCO3��MgCO3,������ָ����55%�����ҵ���[�ɱ�ʾΪSi(Fe)]������ԭ��,���õ���Ʒλ�Ĺ���[�ɱ�ʾΪFe(Si)]����������ͼ����
�Իش���������
��1����Ӧ�٣�����ʯ���յ�CaO��MgO�Ļ�ѧ����ʽΪ�� ��
��2����Ӧ�ڣ���ԭ¯�з�����Ӧ����þ��¯���Ļ�ѧ����ʽΪ�� ��
��3���óɷֵĻ�����һ�ֹ�ҵ��������Ҫԭ�ϣ�����Ϊ���� ԭ�ϡ�
��4���������ط�ұ��þ��һ̨4500ǧ�ߵ�¯�ӿ��ղ�Լ7.2��þ��һ������Լ���ĺ���60%�Ĺ��� �֡�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com