
��2008����ҹ��Ϸ������ı�ѩ�ֺ��У�ʹ����һ����ѩ��������Ҫ�ɷֵĻ�ѧʽΪXY��X��Y��ΪԪ�����ڱ�ǰ20��Ԫ�أ��������Ӻ������ӷֱ���10���Ӻ�18���ӵĵ������ӣ���1molXY����28mol���ӡ�
�Ÿ���ѩ���Ļ�ѧʽ��__ ____��X����Ԫ���γɵĻ�����ĵ���ʽ��____________��
��Ԫ��D��Eԭ�ӵ�����������������Ӳ�����2����D��Y���ڣ���D����ͬ����Ԫ�ص��⻯���У��е���͵���________����ѧʽ����E��ԭ�ӽṹʾ��ͼ��_____ _____��
��Ԫ��R��Yͬ���壬���⻯�������ڿ�ʴ������R2��H2O��Һ��Ӧ�IJ���֮һ��O2���÷�Ӧ�Ļ�ѧ����ʽΪ_______________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
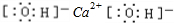
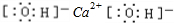
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
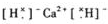
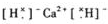
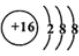
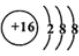
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


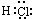
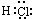
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(8��)��2008����ҹ��Ϸ������ı�ѩ�ֺ��У�ʹ����һ����ѩ��������Ҫ�ɷֵĻ�ѧʽΪXY2��X��Y��Ϊ���ڱ�ǰ20��Ԫ�أ��������Ӻ������ӵĵ��Ӳ�ṹ��ͬ����1mol XY2����54 mol���ӡ�
(1)����ѩ���Ļ�ѧʽ��__________��X����Ԫ���γɵĻ�����ĵ���ʽ��__________��
(2)Ԫ��D��Eԭ�ӵ�����������������Ӳ�����2����D��Y���ڣ���D�����ӽṹʾ��ͼ��__________��D��E���γ�һ�ַǼ��Է��ӣ��÷��ӵĽṹʽΪ__________��D������Ԫ�ص��⻯���У��е���͵���__________��
(3)Ԫ��W��Yͬ���ڣ��䵥����ԭ�Ӿ��壻Ԫ��Z�ĵ��ʷ���Z2����3�����۽���W��Z���γ�һ���������ǽ������ϣ��仯ѧʽ��__________��
(4)Ԫ��R��Yͬ���壬���⻯�������ڿ�ʴ������R2��NaOH��Һ��Ӧ�IJ���֮һ��OR2���÷�Ӧ�����ӷ���ʽΪ
________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com