
��9�֣�����1���±��Dz��ֶ�����Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼ�
|
Ԫ�ش��� |
L |
M |
Q |
R |
T |
|
ԭ�Ӱ뾶/nm |
0.160 |
0.143 |
0.112 |
0.104 |
0.066 |
|
��Ҫ���ϼ� |
+2 |
+3[ |
+2 |
+6��-2 |
-2 |
��LԪ�ص�������______��RԪ�ص�������______��
������TԪ����ɵĻ�����������Ե���________________��д��ѧʽ����
��2��ij����ѩ����Ҫ�ɷ�ΪXY2��X��Y��Ϊ���ڱ���ǰ20��Ԫ�أ��������Ӻ������ӵĵ��Ӳ�ṹ��ͬ����1molXY2����54mol���ӡ�
�ٸ���ѩ����Ҫ�ɷֵĻ�ѧʽΪ__________��X����Ԫ���γɵĻ�����ĵ���ʽΪ____________________��
��Ԫ��D��Eԭ�ӵ���������������Ӧԭ�ӵ��Ӳ�����2����D��Y���ڣ�DԪ����______��EԪ����______��
��1����þ����2�֣� ��BeO��Al2O3 ��2�֣�
��2����CaCl2��1�֣���[H��]-Ca2+[��H]- ��2�֣���S������C����̼����2�֣�
����������1������Ԫ�ص���Ҫ���ϼۺ�ԭ�Ӱ뾶��֪��L��Mg��M��Al��Q��Be��R��S��T��O����������ڶԽ����ϣ��������������Ƶġ�
��2��1molXY2����54mol���ӣ��������Ӻ������ӵĵ��Ӳ�ṹ��ͬ���������ӵ�����������18����X��Ca��Y��Cl�����ǻ��õĽ�������������γ����ӻ������⻯�ƣ�����ʽΪ[H��]-Ca2+[��H]- ����������������Ӧԭ�ӵ��Ӳ�����2�����������C��S������ΪD��Y���ڣ�����D��S��E��C��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�ش��� | W | R | X | Y | Z | Q | M |
| ԭ�Ӱ뾶/nm | 0.037 | 0.186 | 0.074 | 0.075 | 0.077 | 0.110 | 0.143 |
| ��Ҫ���ϼ� | +1 | +1 | -2 | +5��-3 | +4��-4 | +5��-3 | +3 |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꺣��ʡ������ѧ��һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
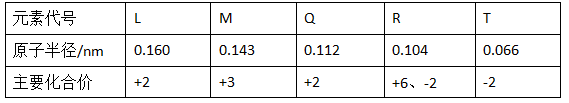
��9�֣�����1���±��Dz��ֶ�����Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼ�
| Ԫ�ش��� | L | M | Q | R | T |
| ԭ�Ӱ뾶/nm | 0.160 | 0.143 | 0.112 | 0.104 | 0.066 |
| ��Ҫ���ϼ� | +2 | +3[ | +2 | +6��-2 | -2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1���±��Dz��ֶ�����Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼ�
| Ԫ�ش��� | L | M | Q | R | T |
| ԭ�Ӱ뾶/nm | 0.160 | 0.143 | 0.112 | 0.104 | 0.066 |
| ��Ҫ���ϼ� | +2 | +3[ | +2 | +6��-2 | -2 |
��LԪ�ص�������________��RԪ�ص�������________��
������TԪ����ɵĻ�����������Ե���________��д��ѧʽ����
��2��ij����ѩ����Ҫ�ɷ�ΪXY2��X��Y��Ϊ���ڱ���ǰ20��Ԫ�أ��������Ӻ������ӵĵ��Ӳ�ṹ��ͬ����1mol XY2����54mol���ӡ�
�ٸ���ѩ����Ҫ�ɷֵĻ�ѧʽΪ________��X����Ԫ���γɵĻ�����ĵ���ʽΪ________��
��Ԫ��D��Eԭ�ӵ���������������Ӧԭ�ӵ��Ӳ�����2����D��Y���ڣ�DԪ����________��EԪ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������ ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com