
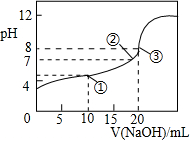
 25”ꏱ£¬Ļņ20.00mL0.1mol•L-1HClO2ČÜŅŗÖŠÖšµĪ¼ÓČė0.1mol•L-1µÄNaOHČÜŅŗ£®ČÜŅŗµÄpHÓėµĪČėNaOHČÜŅŗµÄĢå»żµÄ¹ŲĻµČēĶ¼ĖłŹ¾£¬ŌņĻĀĮŠĖµ·ØÖŠ²»ÕżČ·µÄŹĒ£Ø””””£©
25”ꏱ£¬Ļņ20.00mL0.1mol•L-1HClO2ČÜŅŗÖŠÖšµĪ¼ÓČė0.1mol•L-1µÄNaOHČÜŅŗ£®ČÜŅŗµÄpHÓėµĪČėNaOHČÜŅŗµÄĢå»żµÄ¹ŲĻµČēĶ¼ĖłŹ¾£¬ŌņĻĀĮŠĖµ·ØÖŠ²»ÕżČ·µÄŹĒ£Ø””””£©| A£® | ¢ŁµćŹ±£ŗc£ØClO2-£©£¾c£ØNa+£©£¾c£ØH+£©£¾c£ØOH-£© | |
| B£® | ¢ŚµćŹ±£ŗc£ØNa+£©=c£ØClO2-£© | |
| C£® | ¢ŪµćŹ±£ŗc£ØH+£©=c£ØOH-£©+c£ØHClO2£© | |
| D£® | µĪ¶Ø¹ż³ĢÖŠæÉÄܳöĻÖ£ŗc£ØNa+£©£¾c£ØClO2-£©£¾c£ØOH-£©£¾c£ØH+£© |
·ÖĪö A£®¢ŁµćŹ±£ŗČÜŅŗµÄpHŠ”ÓŚ7£¬ČÜŅŗĻŌĖįŠŌ£»
B£®¢ŚµćŹ±£¬pH=7£¬½įŗĻµēŗÉŹŲŗć·ÖĪö£»
C£®Ļņ20.00mL0.1mol•L-1HClO2ČÜŅŗÖŠÖšµĪ¼ÓČė0.1mol•L-1µÄNaOHČÜŅŗ20mL£¬Ē”ŗĆ·“Ӧɜ³ÉNaClO2£¬øł¾ŻÖŹ×ÓŹŲŗć·ÖĪö£»
D£®µ±Ē”ŗĆÉś³ÉNaClO2Ź±£¬NaClO2Ė®½āČÜŅŗĻŌ¼īŠŌ£®
½ā“š ½ā£ŗA£®¢ŁµćŹ±£ŗČÜŅŗµÄpHŠ”ÓŚ7£¬ČÜŅŗĻŌĖįŠŌ£¬Ōņc£ØH+£©£¾c£ØOH-£©£¬ÓɵēŗÉŹŲŗćc£ØClO2-£©+c£ØOH-£©=c£ØNa+£©+c£ØH+£©æÉÖŖ£¬c£ØClO2-£©£¾c£ØNa+£©£¬ŌņČÜŅŗÖŠĄė×ÓÅØ¶Č¹ŲĻµĪŖ£ŗc£ØClO2-£©£¾c£ØNa+£©£¾c£ØH+£©£¾c£ØOH-£©£¬¹ŹAÕżČ·£»
B£®¢ŚµćŹ±£¬pH=7£¬c£ØH+£©=c£ØOH-£©£¬ÓɵēŗÉŹŲŗćc£ØClO2-£©+c£ØOH-£©=c£ØNa+£©+c£ØH+£©æÉÖŖ£¬c£ØClO2-£©=c£ØNa+£©£¬¹ŹBÕżČ·£»
C£®Ļņ20.00mL0.1mol•L-1HClO2ČÜŅŗÖŠÖšµĪ¼ÓČė0.1mol•L-1µÄNaOHČÜŅŗ20mL£¬Ē”ŗĆ·“Ӧɜ³ÉNaClO2£¬ČÜŅŗÖŠÖŹ×ÓŹŲŗćĪŖ£ŗc£ØOH-£©=c£ØH+£©+c£ØHClO2£©£¬¹ŹC“ķĪó£»
D£®µ±Ē”ŗĆÉś³ÉNaClO2Ź±£¬NaClO2Ė®½āČÜŅŗĻŌ¼īŠŌ£¬c£ØOH-£©£¾c£ØH+£©£¬ClO2-·¢ÉśĖ®½āÅØ¶Č¼õŠ”£¬ĖłŅŌc£ØNa+£©£¾c£ØClO2-£©£¾c£ØOH-£©£¾c£ØH+£©£¬¹ŹDÕżČ·£®
¹ŹŃ”C£®
µćĘĄ ±¾Ģāæ¼²éĖį¼ī»ģŗĻµÄ¶ØŠŌÅŠ¶Ļ¼°ČÜŅŗpHµÄ¼ĘĖć£¬ĢāÄæÄѶČÖŠµČ£¬Ć÷Č·ø÷µć¶ŌÓ¦ČÜÖŹ×é³ÉĪŖ½ā“š¹Ų¼ü£¬×¢ŅāÕĘĪÕµēŗÉŹŲŗć”¢ĪļĮĻŹŲŗć¼°ŃĪµÄĖ®½āŌĄķŌŚÅŠ¶ĻĄė×ÓÅØ¶Č“óŠ”ÖŠµÄÓ¦ÓĆ£¬ŹŌĢāÅąŃųĮĖѧɜµÄ·ÖĪö”¢Ąķ½āÄÜĮ¦¼°Įé»īÓ¦ÓĆÄÜĮ¦£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | D2O·Ö×ÓÖŠŃõŌ×ÓĪŖsp3ŌÓ»Æ | |
| B£® | CrŌ×Ó¼Ūµē×ÓÅŲ¼Ź½£ŗ3d54s1 | |
| C£® | ĮņĄė×ÓµÄŗĖĶāµē×ÓÅŲ¼Ź½£ŗ1s22s22p63s23p6 | |
| D£® | SŌ×ӵĵē×ÓÅŲ¼Ķ¼£ŗ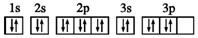 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | FeCl3 | B£® | Na2S | C£® | £ØNH4£©2CO3 | D£® | Na2SO4 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| Ń”Ļī | ŹµŃé²Ł×÷ | ĻÖĻó | ½āŹĶ |
| A | ŌŚĆܱÕČŻĘ÷ÖŠ¼ÓČČCuOÖĘ1000”ę | ŗŚÉ«¹ĢĢå±ä³ÉŗģÉ«¹ĢĢå | CuOŹÜČČ·Ö½āµĆµ½µ„ÖŹCu |
| B | ½«SO2ĶØČėĘ·ŗģČÜŅŗÖŠ | ČÜŅŗĶŹÉ« | SO2¾ßÓŠĘư׊Ō |
| C | ½«Mg”¢AlÓėNaOHČÜŅŗ×é³ÉŌµē³Ų | Alµē¼«Čܽā | Al±ČMg½šŹō»ī¶ÆŠŌĒæ |
| D | ĻņijČÜŅŗÖŠ¼ÓČėŃĪĖįĖį»ÆµÄĀČ»Æ±µČÜŅŗ | ÓŠ°×É«³ĮµķÉś³É | øĆČÜŅŗÖŠŅ»¶Øŗ¬ÓŠSO42- |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | øĆ·“Ó¦Ö¤Ć÷ĮĖ£¬ŗ¬×īøß¼ŪŌŖĖŲµÄ»ÆŗĻĪļ£¬Ņ»¶Ø¾ßÓŠĒæŃõ»ÆŠŌ | |
| B£® | 1mol»¹Ō¼Į·“Ó¦Ź±£¬×ŖŅʵĵē×ÓŹżĪŖ2NA | |
| C£® | H2SO4ŌŚ·“Ó¦ÖŠ±ķĻÖĮĖŃõ»ÆŠŌŗĶĖįŠŌ | |
| D£® | æÉŅŌĄūÓĆøĆ·“Ó¦ŌĄķ£¬½«ĖüÉč¼Ę³ÉŌµē³Ų£¬Ķعż¼ģ²āµēĮ÷Ēæ¶ČÅŠ¶ĻĖ¾»śŹĒ·ńŅū¾Ę |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com