
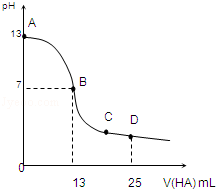
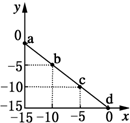
 �����£���25mL 0.1mol/L MOH��Һ����μ���0.2mol/L HA��Һ��������ͼ��ʾ������仯���Բ��ƣ����ش��������⣺
�����£���25mL 0.1mol/L MOH��Һ����μ���0.2mol/L HA��Һ��������ͼ��ʾ������仯���Բ��ƣ����ش��������⣺���� ��1����ͼ���֪0.1mol/L MOH��Һ��pH=13��c��OH-��=0.1mol/L��˵��Ϊǿ���ȫ���룻
��2����ͼ���֪��������13mL 0.2mol/L HA��Һʱ��n��HA��=0.0026mol����n��MOH��=0.0025mol��˵��HA����������Һ�����ԣ�˵��HAΪ���ᣬ�����ǡ�÷�Ӧ��Ӧ����ǿ�������Σ��ٽ�ˮ�ĵ��룻
��3��B����Һ�����ԣ���ϵ���غ��жϣ�
��4��D�㷴Ӧ�õ���Ũ�ȵ�MA��HA��Һ����������غ��жϣ�
��� �⣺��1����ͼ���֪0.1mol/L MOH��Һ��pH=13��c��OH-��=0.1mol/L��˵��Ϊǿ���ȫ���룬����뷽��ʽΪMOH�TM++OH-��
�ʴ�Ϊ��MOH�TM++OH-��
��2����ͼ���֪��������13mL 0.2mol/L HA��Һʱ��n��HA��=0.0026mol����n��MOH��=0.0025mol��˵��HA����������Һ�����ԣ�˵��HAΪ���ᣬ�����ǡ�÷�Ӧ��Ӧ����ǿ�������Σ�ˮ��ʼ��ԣ�����A-+H2O?HA+OH-���ٽ�ˮ�ĵ��룬������Һ����ˮ�������c��H+������0.2mol/L HA��Һ����ˮ�������c��H+����
�ʴ�Ϊ���A-+H2O?HA+OH -������
��3��B����Һ�����ԣ���c��H+��=c��OH-������ϵ���غ㣺c��M+��+c��H+��=c��A-��+c��OH-������֪��c��M+��=c��A-������������Ũ��ԶԶ����������Ũ�ȣ���Һ�д���c��M+��=c��A-����c��H+��=c��OH-����
�ʴ�Ϊ��c��M+��=c��A-����c��H+��=c��OH-����
��4��D�㷴Ӧ�õ���Ũ�ȵ�MA��HA��Һ��AԪ����A-��HA������ʽ���ڣ���Mȫ��M+��ʽ���ڣ��������غ�ɵã�c��A-��+c��HA��=2c��M+����
�ʴ�Ϊ��=��
���� ���⿼�����������Һ�����жϡ�����ˮ�⡢����Ũ�ȴ�С�Ƚϵȣ�������ѧ���ķ��������Ŀ��飬��ȷͼ���и�����ĺ����ǽⱾ��ؼ���ע�����غ㡢�����غ�������Ũ�ȵ�����ϵ�Ƚ���Ӧ�ã�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ש���е���Ҫ�ɷ��ǹ����� | |
| B�� | ��ש�е���Ԫ����Ҫ����������������ʽ���� | |
| C�� | ��ש�е���Ԫ����Ҫ������������ʽ���� | |
| D�� | ��ש�е���Ԫ����Ҫ��������������ʽ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
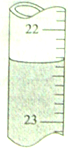 ȷ��ȡ25.00mLijδ֪Ũ�ȵ�NaOH��Һ��һ�ྻ��ƿ�У�Ȼ����0.20mol/L��������Һ�ζ���ָʾ��Ϊ���ȣ����ζ�������£�
ȷ��ȡ25.00mLijδ֪Ũ�ȵ�NaOH��Һ��һ�ྻ��ƿ�У�Ȼ����0.20mol/L��������Һ�ζ���ָʾ��Ϊ���ȣ����ζ�������£�| HCl��Һ��ʼ���� | HCl��Һ�յ���� | |
| ��һ�� | 2.15mL | |
| �ڶ��� | 3.10mL | 21.85mL |
| ������ | 4.20mL | 22.95mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
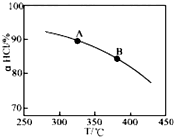 ��O2��HClת��ΪCl2�������Ч�棬������Ⱦ��
��O2��HClת��ΪCl2�������Ч�棬������Ⱦ��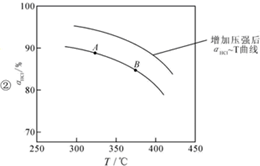 ����ѹǿ��ƽ�����ƣ���HCl������ͬ�¶��£�HCl��ƽ��ת���ʱ�֮ǰʵ��Ĵ�
����ѹǿ��ƽ�����ƣ���HCl������ͬ�¶��£�HCl��ƽ��ת���ʱ�֮ǰʵ��Ĵ�| t��min�� | 0 | 2.0 | 4.0 | 6.0 | 8.0 |
| n��Cl2��/10-3mol | 0 | 1.8 | 3.7 | 5.4 | 7.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ij�¶��µ���Һ��c��H+��=10xmol/L��c��OH-��=10ymol/L��x��y�Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
ij�¶��µ���Һ��c��H+��=10xmol/L��c��OH-��=10ymol/L��x��y�Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ���¶ȸ���25�� | |
| B�� | ͼ��a����Һ�ʼ��� | |
| C�� | ���¶��£�0.01 mol•L-1��HCl��Һ�У���ˮ�������H+Ũ��Ϊ10-12 mol•L-1 | |
| D�� | ���¶��£������Ũ�Ⱦ�Ϊ0.01 mol•L-1��HCl��Һ��NaOH��Һǡ����ȫ��Ӧ��pH=7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��CH3COO-����c��Na+�� | B�� | c��CH3COOH����c��CH3COO-�� | ||
| C�� | 2c��H+��=c��CH3COO-��-c��CH3COOH�� | D�� | c��CH3COOH��+c��CH3COO-��=0.01mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��Ϻ����Һ | ������ᡡ | �����Ũ��/��mol•L-1���� | ����Ϻ���Һ��pH |
| ���� | ��HA | 0.10�� | ��8.7 |
| ���� | HB | 0.12 | 2 |
| A�� | HA��ǿ�ᣬHB������ | |
| B�� | �����¶ȣ���Һ����$\frac{c��{B}^{-}��}{c��N{a}^{+}��}$���� | |
| C�� | ��Һ��������Ũ�ȵĹ�ϵ��c��A-����c��Na+����c��OH-����c��H+�� | |
| D�� | ��Һ��������Ũ�ȵĹ�ϵ��c��Na+��+c��H+��+c��B-��=0.12 mol•L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ�� ��� | HA���ʵ��� Ũ�ȣ�mol• L-1�� | NaOH���ʵ� ��Ũ�ȣ�mol• L-1�� | �����Һ�� pH |
| �� | 0.2 | 0.2 | pH=a |
| �� | c1 | 0.2 | pH=7 |
| �� | 0.2 | 0.1 | pH��7 |
| �� | 0.1 | 0.1 | pH=9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 4�� | B�� | 5�� | C�� | 6�� | D�� | 7�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com