
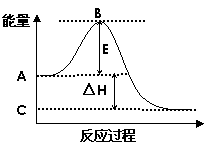
 £Ø£±£©£Ø4·Ö£©2SO2(g)+O2(g)
£Ø£±£©£Ø4·Ö£©2SO2(g)+O2(g) ![]() 2SO3(g)·“Ó¦¹ż³ĢµÄÄÜĮæ±ä»ÆČēĶ¼ĖłŹ¾”£ŅŃÖŖ1mol SO2(g)Ńõ»ÆĪŖ1mol SO3(g)µÄ¦¤H= ”Ŗ99kJ£Æmol”£
2SO3(g)·“Ó¦¹ż³ĢµÄÄÜĮæ±ä»ÆČēĶ¼ĖłŹ¾”£ŅŃÖŖ1mol SO2(g)Ńõ»ÆĪŖ1mol SO3(g)µÄ¦¤H= ”Ŗ99kJ£Æmol”£
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
![]() ¢ŁĶ¼ÖŠAµć±ķŹ¾£ŗ
¢ŁĶ¼ÖŠAµć±ķŹ¾£ŗ
Cµć±ķŹ¾£ŗ
EµÄ“󊔶ŌøĆ·“Ó¦µÄ·“Ó¦ČČ £ØĢī”°ÓŠ”±»ņ”°ĪŽ”±£©Ó°Ļģ”£
![]() ¢ŚĶ¼ÖŠ”÷H= kJ/mol”£
¢ŚĶ¼ÖŠ”÷H= kJ/mol”£
£Ø£²£©£Ø4·Ö£©ÓÉĒāĘųŗĶŃõĘų·“Ӧɜ³É1 molĖ®ÕōĘų£¬·Å³ö241.8 kJČČĮæ(25”ę”¢101 kPaĻĀ²āµĆ)
¢ŁŠ“³öøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½£ŗ
¢ŚČō1 molĖ®ÕōĘų×Ŗ»ÆĪŖŅŗĢ¬Ė®·ÅČČ45kJ£¬Ōņ·“Ó¦H2(g) £«![]() O2(g) £½ H2O( l )µÄ
O2(g) £½ H2O( l )µÄ
¦¤H = kJ/mol”£ĒāĘųµÄČ¼ÉÕČČĪŖ¦¤H = kJ/mol”£
£Ø£±£©£Ø4·Ö£©¢Ł![]() ·“Ó¦ĪļµÄ×ÜÄÜĮ棻 Éś³ÉĪļµÄ×ÜÄÜĮ棻 ĪŽ£» £198 ”££ØĆææÕ£±·Ö£©
·“Ó¦ĪļµÄ×ÜÄÜĮ棻 Éś³ÉĪļµÄ×ÜÄÜĮ棻 ĪŽ£» £198 ”££ØĆææÕ£±·Ö£©
£Ø£²£©£Ø4·Ö£©H2 ( g ) +![]() O2 ( g )£½H2O ( g ) £Ø£±·Ö£©”” ¦¤H £½ØD241.8 kJ/mol £Ø£±·Ö£¬²»Š“µ„Ī»ĪŖ0·Ö£©”£ £Ø »ņ2H2 (g) +O2(g)£½2H2O(g) ””””””¦¤H £½ØD483.6kJ/mol £©”£
O2 ( g )£½H2O ( g ) £Ø£±·Ö£©”” ¦¤H £½ØD241.8 kJ/mol £Ø£±·Ö£¬²»Š“µ„Ī»ĪŖ0·Ö£©”£ £Ø »ņ2H2 (g) +O2(g)£½2H2O(g) ””””””¦¤H £½ØD483.6kJ/mol £©”£
ØD286.8 £Ø£±·Ö£©£» ØD286.8 £Ø£±·Ö£©””””””””””””



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚŅ»Ćܱյģ²£ĢµÄČŻĘ÷Ąļ³äČė8mol SO2ŗĶ£“mol 18O2£¬ŌŚŅ»¶ØĢõ¼žĻĀæŖŹ¼·“Ó¦£ŗ2SO2£Øg£©£«O2£Øg£©![]() 2SO3£Øg£©
2SO3£Øg£©
![]() 2minÄ©²āµĆČŻĘ÷ÖŠÓŠ7.2mol SO2”£
2minÄ©²āµĆČŻĘ÷ÖŠÓŠ7.2mol SO2”£
![]() ŹŌ»Ų“š£ŗ
ŹŌ»Ų“š£ŗ
![]() £Ø£±£©·“Ó¦ŗó18£Ļ“ęŌŚÄÄŠ©ĪļÖŹÖŠ””””””””””””””””””””””””””””””””””£»
£Ø£±£©·“Ó¦ŗó18£Ļ“ęŌŚÄÄŠ©ĪļÖŹÖŠ””””””””””””””””””””””””””””””””””£»
![]() £Ø£²£©2minÄ©SO3µÄÅØ¶Č£»
£Ø£²£©2minÄ©SO3µÄÅØ¶Č£»
![]() £Ø£³£©ÓĆO2µÄÅØ¶Č±ä»Æ±ķŹ¾øĆŹ±¼ä¶ĪÄŚµÄ»Æѧ·“Ó¦ĖŁĀŹ”£
£Ø£³£©ÓĆO2µÄÅØ¶Č±ä»Æ±ķŹ¾øĆŹ±¼ä¶ĪÄŚµÄ»Æѧ·“Ó¦ĖŁĀŹ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø6·Ö£©ŌŚŅ»Ćܱյģ²£ĢµÄČŻĘ÷Ąļ³äČė8mol SO2ŗĶ£“mol 18O2£¬ŌŚŅ»¶ØĢõ¼žĻĀæŖŹ¼·“Ó¦£ŗ2SO2£Øg£©£«O2£Øg£©![]() 2SO3£Øg£©
2SO3£Øg£©
2minÄ©²āµĆČŻĘ÷ÖŠÓŠ7.2mol SO2”£ŹŌ»Ų“š£ŗ
£Ø£±£©·“Ó¦ŗó18£Ļ“ęŌŚÄÄŠ©ĪļÖŹÖŠ””””””””””””””””””””””””””””””””””£»
£Ø£²£©2minÄ©SO3µÄÅØ¶Č£»
£Ø£³£©ÓĆO2µÄÅØ¶Č±ä»Æ±ķŹ¾øĆŹ±¼ä¶ĪÄŚµÄ»Æѧ·“Ó¦ĖŁĀŹ”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com