
��һʵ�����ͼ��ʾ����30 mL 0.1 mol/L Ba(OH)2��Һ�����ձ��У�Ȼ��߽������������0.1 mol/Lij��3����Ԫ���γɵ�ij����Һ��25 mL����������Һ�����V�͵���ǿ��I��I��Vͼ��ͼ����ʾ��
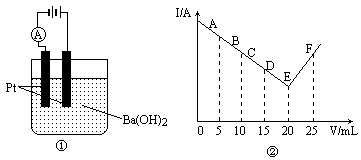
(1)����Ļ�ѧʽΪ________��
(2)����C��ʱ����Һ�е������������Ҫ��________��
(3)����F��ʱ����Һ�е������������Ҫ��________��
(4)�ձ��иռ�����ʱ���۲쵽��������________��
(5)ͼ���У���A��E����ǿ��I��С����Ҫԭ����________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
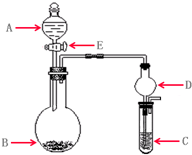 ��2011?������ģ�⣩ʵ������Ҫ����ijЩ����ʱ��ͨ��ʹ�ÿ��ٵķ����Ʊ������м���ʵ��ɿ�����ȡʵ����������������壬�������������ʵ�飮��ʵ��װ����ͼ��ʾ��
��2011?������ģ�⣩ʵ������Ҫ����ijЩ����ʱ��ͨ��ʹ�ÿ��ٵķ����Ʊ������м���ʵ��ɿ�����ȡʵ����������������壬�������������ʵ�飮��ʵ��װ����ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2012?��ɳģ�⣩ijʵ��С��ͬѧΪ��̽��ͭ��Ũ����ķ�Ӧ������������ϵ��ʵ�飮
��2012?��ɳģ�⣩ijʵ��С��ͬѧΪ��̽��ͭ��Ũ����ķ�Ӧ������������ϵ��ʵ�飮
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2008?��ׯһģ����V1mL 1.00mol?L-1�����V2mLδ֪Ũ��NaOH��Һ��Ͼ��Ⱥ��������¼��Һ�¶ȣ�ʵ������ͼ��ʾ��ʵ����ʼ�ձ���V1+V2=50mL�������������У���ȷ���ǣ�������
��2008?��ׯһģ����V1mL 1.00mol?L-1�����V2mLδ֪Ũ��NaOH��Һ��Ͼ��Ⱥ��������¼��Һ�¶ȣ�ʵ������ͼ��ʾ��ʵ����ʼ�ձ���V1+V2=50mL�������������У���ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
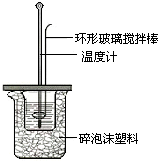
|
�¶� ʵ����� |
��ʼ�¶�t1/�� |
��ֹ�¶� t2/�� |
�¶Ȳ�ƽ��ֵ ��t2-t1��/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 30.1 | 4.0 4.0 |
| 2 | 27.0 | 27.4 | 27.2 | 33.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
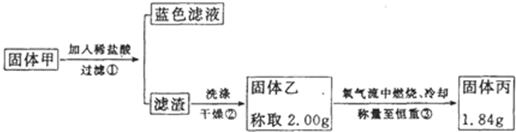
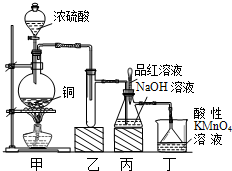 ʵ������Ũ������ͭ�ķ�Ӧ��ȡ����NaHSO3��ʵ��װ����ͼ��ʾ��
ʵ������Ũ������ͭ�ķ�Ӧ��ȡ����NaHSO3��ʵ��װ����ͼ��ʾ��| ʵ����� | Ԥ����������� |
| ����1��ȡ��������Һ�����Թ��У��μӹ���lmol/L�Ȼ�����Һ������һ��ʱ��õ���ҺA����B�� | / |
| ����2��������B�м�������ˮϴ�ӳ��������ú���ȥ�ϲ���Һ����������2�Σ���������Ʒ�죬�� |
��Ʒ����ɫ���������ݣ����� |
| ����3�� |
�� �� ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com