
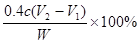
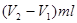 £¬ĖłŅŌŌѳʷ֊ĒāŃõ»ÆÄʵÄÖŹĮæŹĒ
£¬ĖłŅŌŌѳʷ֊ĒāŃõ»ÆÄʵÄÖŹĮæŹĒ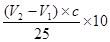 £¬ĖłŅŌÉÕ¼īѳʷ“æ¶ČµÄ¼ĘĖćŹ½ĪŖ
£¬ĖłŅŌÉÕ¼īѳʷ“æ¶ČµÄ¼ĘĖćŹ½ĪŖ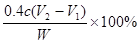 ”£
ӣ


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
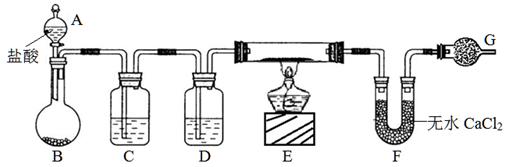
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗ¼ĘĖćĢā
| Ļ”ĮņĖįÓĆĮæ | Ź£Óą¹ĢĢåÖŹĮæ |
| µŚŅ»“Ī¼ÓČė10g | mg |
| µŚ¶ž“Ī¼ÓČė10g | 2.0g |
| µŚČż“Ī¼ÓČė10g | 1.5g |
| µŚĖÄ“Ī¼ÓČė10g | 1.0g |
| µŚĪå“Ī¼ÓČė10g | 0.6g |
| µŚĮł“Ī¼ÓČė10g | 0.6g |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
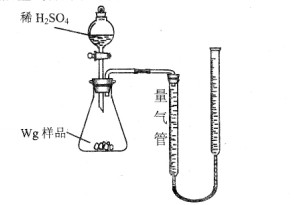
| A£®ŃĪĖį | B£®KSCNČÜŅŗ | C£®ĖįŠŌKMnO4ČÜŅŗ | D£®H2O2ČÜŅŗ |
| ŠņŗÅ | ČÜŅŗÖŠæÉÄÜ“ęŌŚ µÄ½šŹōĄė×Ó | Ń”Ōń×īÉŁÖÖŹżµÄŹŌ¼Į£¬ŃéÖ¤øĆ ¼ŁÉč(Ģī×ÖÄø) |
| ¢Ł | | |
| ¢Ś | | |
| ¢Ū | | |
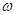 (Fe) (b-a)”Į10-3L
(Fe) (b-a)”Į10-3L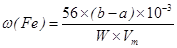
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
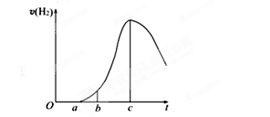
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
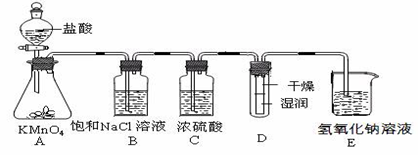
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com