
ij100mL��Һ�н����±������е�5�����ӣ�������ˮ�ĵ��뼰���ӵ�ˮ�⣩���Ҹ����ӵ����ʵ�����Ϊ0.1mol��
|
������ |
|
|
������ |
|
������ԭ��Һ�м���KSCN��Һ�������Ա仯��
������ԭ��Һ�м�����������ᣬ���������ɣ���Һ������������䡣
������ԭ��Һ�м���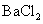 ��Һ���а�ɫ�������ɡ�
��Һ���а�ɫ�������ɡ�
�Իش��������⣺
��1��ԭ��Һ�������������ǣ�д���ӷ��ţ���ͬ�� ���������� ��
��2������ԭ��Һ���ȼ����������ᣬ�ٵ���KSCN��Һ��ʵ������������Ӧ�����ӷ���ʽ�� �� ��
��3������ԭ��Һ�м�������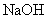 ��Һ����ַ�Ӧ����һ��ʱ�䣬���ˡ�ϴ�ӡ����գ��������ù����������
��
��Һ����ַ�Ӧ����һ��ʱ�䣬���ˡ�ϴ�ӡ����գ��������ù����������
��
��4����ԭ��Һ�м����������������ˮ���ռ������������岢ʹ����ǡ�ó����������Խ�����������ˮ���У�����������ͨ�� mL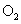 �����������ָ��״��������ʹ��Һ������������
�����������ָ��״��������ʹ��Һ������������
 �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ������ | SO42-��NO3-��Cl- |
| ������ | Fe3+��Fe2+��NH4+��Cu2+��Al3+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ������ | SO42-��NO3-��Cl- |
| ������ | Fe3+��Fe2+��NH4+��Cu2+��Al3+ |
| 1 |
| 22.4 |
| 1 |
| 22.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���㽭ʡ�����и������ϣ����л�ѧ�Ծ��������棩 ���ͣ������
| ������ | SO42-��NO3-��Cl- |
| ������ | Fe3+��Fe2+��NH4+��Cu2+��Al3+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���㽭ʡ���������������ϣ����л�ѧ�Ծ��������棩 ���ͣ������
| ������ | SO42-��NO3-��Cl- |
| ������ | Fe3+��Fe2+��NH4+��Cu2+��Al3+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������ʡ��ɽһ�и߿���ѧ��ģ�Ծ��������棩 ���ͣ������
| ������ | SO42-��NO3-��Cl- |
| ������ | Fe3+��Fe2+��NH4+��Cu2+��Al3+ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com