
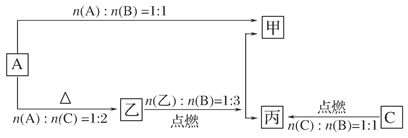
 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ______������ţ�����ȱ�ٵ������� ��
______������ţ�����ȱ�ٵ������� ��| A����30mLˮϴ���ձ�2~3�Σ�ϴ��Һ��ע������ƿ |
| B����������ƽȷ��ȡ�����Na2CO3?10H2O����������������ձ��У��ټ�������ˮ��Լ30mL�����ò���������������ʹ�����ܽ� |
| C��������ȴ����Һ�ز�����ע��250mL������ƿ�� |
| D��������ƿ�ǽ�����ҡ�� |

�鿴�𰸺ͽ���>>
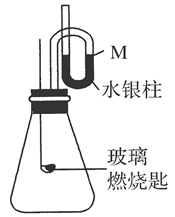
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
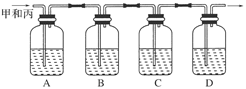
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
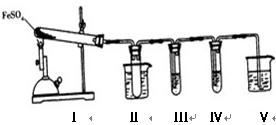
| �����Լ� | Ԥ����������� |
| װ�â���Թ��м��� �� | ����������ɫ������֤����������к���SO3 |
| װ�â����Թ��м��� �� |  |
 ����������̽����ʵ���������ɷ֡������ʵ����ƣ���д�����Լ���Ԥ����������ۡ���ѡ�Լ���3mol��L��1H2SO4��6 mol��L��1NaOH��0.5 mol��L��1BaCl2��0.5 mol��
����������̽����ʵ���������ɷ֡������ʵ����ƣ���д�����Լ���Ԥ����������ۡ���ѡ�Լ���3mol��L��1H2SO4��6 mol��L��1NaOH��0.5 mol��L��1BaCl2��0.5 mol�� L��1Ba(NO3)2��0.01 mol��L��1����KMnO4��Һ��0.01 mol��L��1��ˮ
L��1Ba(NO3)2��0.01 mol��L��1����KMnO4��Һ��0.01 mol��L��1��ˮ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
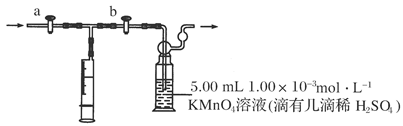
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 CO�� + CO2�� + H2O��
CO�� + CO2�� + H2O��
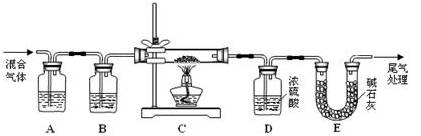
�鿴�𰸺ͽ���>>
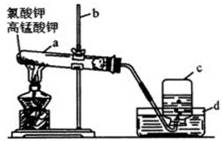
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
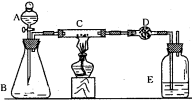
 װ�ÿ�����_____________������ʢ�ŵ�ҩƷ��____________��
װ�ÿ�����_____________������ʢ�ŵ�ҩƷ��____________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
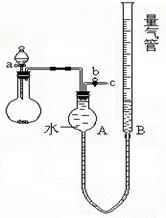
| | ���ʹ������� | �����Ƶ����� | �����ܵ�һ�ζ��� | �����ܵڶ��ζ��� |
| �� | 0.62 g | 5 .0g(����) | 40 mL | 264 mL |
| �� | 0.31 g | 2.5 g(����) | 40 mL | 152mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������������е���Ԫ��ʱ�����������NaOH��Һ��ϼ��Ⱥ���ϡ��������ữ |
| B��Ϊ��߸��������Һ�����������������Ὣ���������Һ�����ữ |
| C����ȥ���еļױ������μӸ��������Һ��NaOH��Һ��Ȼ���÷�Һ©����Һ |
| D������CH3��CH==CH��CHO������ȩ����������ˮ��������Һ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com