
��������(Na2FeO4)��һ����������������ҵ���Ʊ������������������ַ�����
a��2Fe(OH)3��3NaClO��4NaOH===2Na2FeO4��3NaCl��5H2O��
b��2FeSO4��6Na2O2===2Na2FeO4��2Na2O��2Na2SO4��O2����
c��Fe2O3��3Na2O2===2Na2FeO4��Na2O��
d��Fe(NO3)3��NaOH��Cl2�D��Na2FeO4��NaNO3��NaCl��H2O��
��ش��������⣺
(1)�����ж���ȷ����________(�����)��
A������a��b��c������ˮ��Һ�н���
B������a��b��֪NaClO��Na2O2�������Ծ�ǿ��Na2FeO4��
C��FeSO4ֻ�л�ԭ�ԣ�û��������
D������KSCN��Һ����b�IJ������Ƿ���FeSO4
(2)���ڷ���c����˵����ȷ����________(�����)��
A��Na2O2�������������ǻ�ԭ��
B����ԭ����ֻ��Na2O
C��3 mol Na2O2������Ӧ����6 mol����ת��
D����Na2FeO4��FeΪ��4�ۣ�����ǿ�����ԣ�������ɱ��
(3)���ڷ���d����ش��������⣺
���������뻹ԭ�������ʵ���֮��Ϊ________��
��д��Na2FeO4��H2O��Ӧ�����ӷ���ʽ��____________________________��
�����Ʋ�Na2FeO4����������ɱ���⣬��һ����;��____________________��
������(1)ѡ��A������b��c�ж���Na2O2���뷴Ӧ������ˮ��Һ�н��У�Na2O2��H2O��Ӧ���Ӷ����õ�Na2FeO4��ѡ��B����Ӧ��NaClO��Na2O2����õ��ӣ�Ϊ��������Na2FeO4Ϊ�����������NaClO��Na2O2�������Ծ�ǿ��Na2FeO4�������ԡ�ѡ��C��FeSO4�е�FeԪ��Ϊ��2�ۣ��ڷ�Ӧ�п�ʧȥ���ӣ�Ҳ�ɵõ����ӣ�����FeSO4���������ԣ�Ҳ�л�ԭ�ԡ�ѡ��D��FeSO4������KSCN��Һ��Ӧ����ɫ��Һ��
(2)ѡ��A�����ݷ�Ӧ��Fe2O3��3Na2O2===2Na2FeO4��Na2O��Na2O2�ڷ�Ӧ��ֻ��õ��ӣ�ֻ����������ѡ��B��Na2O2��Ӧ�IJ������Na2FeO4��Na2O�����߶��ǻ�ԭ���ѡ��C,3 mol Na2O2������Ӧ����6 mol����ת�ƣ���ȷ��ѡ��D��Na2FeO4��FeԪ�صĻ��ϼ�Ϊ��6�ۡ�
(3)��������ΪCl2���仯ѧ������Ϊ3����ԭ��ΪFe(NO3)3���仯ѧ������Ϊ2�����ߵ����ʵ���֮��Ϊ3��2���ڸ�����Ŀ��Ϣ����Ӧ��ΪNa2FeO4��H2O��������ΪFe(OH)3��O2��NaOH�����У�Na2FeO4��H2O�D��Fe(OH)3(����)��NaOH��O2������ƽ�ķ�Ӧ��ѧ����ʽΪ4Na2FeO4��10H2O===4Fe(OH)3(����)��8NaOH��3O2���������ӷ�Ӧ����ʽΪ4FeO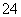 ��10H2O===4Fe(OH)3(����)��8OH����3O2����Na2FeO4����ǿ�����ԣ������������ɵ�Fe(OH)3������нϴ�ı������������ˮ�е��������ˮ�����á�
��10H2O===4Fe(OH)3(����)��8OH����3O2����Na2FeO4����ǿ�����ԣ������������ɵ�Fe(OH)3������нϴ�ı������������ˮ�е��������ˮ�����á�
�𰸡�(1)B��(2)C
(3)��3��2����4FeO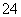 ��10H2O===4Fe(OH)3(����)��8OH����3O2�������ɵ������������壬���нϴ�ı���������������ʶ��ﵽ��ˮ��Ŀ��
��10H2O===4Fe(OH)3(����)��8OH����3O2�������ɵ������������壬���нϴ�ı���������������ʶ��ﵽ��ˮ��Ŀ��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ�������£����ڿ��淴ӦX(g)��3Y(g)2Z(g)����X��Y��Z��ʼŨ�ȷֱ�Ϊc1��c2��c3(����Ϊ��)������ƽ��ʱ��X��Y��Z��Ũ�ȷֱ�Ϊ0.1 mol·L��1��0.3 mol·L��1��0.08 mol·L��1���������жϲ���������(����)
A��c1c2��13
B��ƽ��ʱ��Y��Z����������֮��Ϊ23
C��X��Y��ת�������
D��c1��ȡֵ��ΧΪ0 mol·L��1<c1<0.14 mol·L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ���� (����)��
A����ƹ����У������ǿ�ͨ��ˮ�ⷴӦ���ɾƾ�
B����������Һ�м��뱥����������Һ�����ɵij����ﲻ�����ܽ�
C����ɫ���Ը��������Һ�м���ֲ���ͳ������Һ��ɫ����ȥ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
G(�����ᱡ�ɴ���)��һ���������ಡ��ҩ���ϳ���·���£�
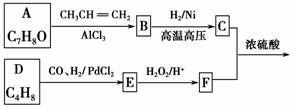


(1)A������Ϊ________��
(2)G�к�������������Ϊ________��
(3)D�ķ����к���________�ֲ�ͬ��ѧ��������ԭ�ӡ�
(4)E�����Ƶ�������ͭ��Ӧ�Ļ�ѧ����ʽΪ__________________________
__________________________________________________________��
(5)д����������������A������ͬ���칹��Ľṹ��ʽ��____________��
a����������6��̼ԭ����һ��ֱ���ϣ�
b���������OH��
(6)����ȩ���������ϡ��ٽ����ȣ�д�����Ҵ�Ϊԭ���Ʊ�CH3(CH2)3CHO�ĺϳ�·������ͼ(���Լ���ѡ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ��������ԭ��Ӧ��ϵ�з�Ӧ������ﹲ�������ӣ�Fe3����NO ��Fe2����NH
��Fe2����NH ��H����H2O������������ȷ���� (����)��
��H����H2O������������ȷ���� (����)��
A���÷�Ӧ˵��Fe(NO3)2��Һ���˼����ữ
B���÷�Ӧ���������뻹ԭ�����ʵ���֮��Ϊ8��1
C������1 mol NO ����������Ӧ��ת�Ƶ���5 mol
����������Ӧ��ת�Ƶ���5 mol
D�������÷�Ӧ��Ƴ�ԭ��أ�����ӦΪFe3����e��===Fe2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ���� (����)��
A����������������ʱ�����������ζ������۶ϣ������ڽ���ԭ��֮���н�
ǿ������
B��ͨ������£�����������ɵ��ӻᷢ�������ƶ����γɵ���
C�������ǽ������ɵ��ӵ��˶������������¶ȸߵIJ��ִ����¶ȵ͵IJ���
D�������ĵ��������¶ȵ����߶�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ͻ�������Ľṹ�����ʣ��ش��������⣺
(1)���������������ж�һ��Ϊ�����������________��
A���ɷ��Ӽ��������γɣ��۵�ܵ�
B���ɹ��ۼ�����γ���״���壬�۵�ܸ�
C�����������õĵ����ԡ������Ժ���չ��
(2)���й��ڽ��������������ȷ����________��
A�������£��������ʶ��Խ���������ʽ����
B�����������������ɵ���֮���ǿ�����ã���һ�����������£������α�
����ʧ
C���Ƶ��ۡ��е���ڼ�
D���¶�Խ�ߣ������ĵ�����Խ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
3��������(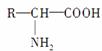 ������R������ͬ��Ҳ���Բ�ͬ)����ʧȥ2��ˮ�������ϳ����ġ����з���ʽΪC36H57O18N11��ʮһ����ȫˮ��ɸʰ���(C2H5O2N)��������(C3H7O2N)���Ȱ���(C5H9O4N)��������ʮһ�Ļ�����ʱ����3�ְ���������ʵ���֮��Ϊ(����)
������R������ͬ��Ҳ���Բ�ͬ)����ʧȥ2��ˮ�������ϳ����ġ����з���ʽΪC36H57O18N11��ʮһ����ȫˮ��ɸʰ���(C2H5O2N)��������(C3H7O2N)���Ȱ���(C5H9O4N)��������ʮһ�Ļ�����ʱ����3�ְ���������ʵ���֮��Ϊ(����)
A��3��3��5 B��3��5��3
C��5��3��3 D��8��7��7
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ��Ӧ��2A��B2C������A��B��C��Ϊ���壬��ͼ�е������Ǹ÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�����ߣ�x���ʾ�¶ȣ�y���ʾB��ת���ʣ�ͼ����a��b��c���㣬��ͼ��ʾ��������������ȷ���� (����)��
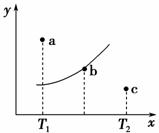
A���÷�Ӧ�Ƿ��ȷ�Ӧ
B��b��ʱ��������ƽ��Ħ���������ٱ仯
C��T1�¶���a���ʾ����ﵽƽ�⣬���Բ�ȡ����ѹǿ�ķ���
D��c��ɱ�ʾv(��)��v(��)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com