

·ÖĪö £Ø1£©ÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗŅ»°ć²½ÖčĪŖ£ŗ¼ĘĖć”¢³ĘĮ攢Čܽā”¢ĄäČ“”¢ŅĘŅŗ”¢Ļ“µÓ”¢¶ØČŻ”¢Ņ”ŌČµČ£¬¾Ż“ĖÅÅŠņ£»
£Ø2£©ŅĄ¾ŻÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗŅ»°ć²½ÖčŃ”ŌńŠčŅŖŅĒĘ÷£»
£Ø3£©ĢģĘ½³ĘĮæĪļĢåŹ±×ńŃ×óĪļÓŅĀėµÄŌŌņ£¬ĢģĘ½Ę½ŗāŌĄķ£ŗ×óÅĢĪļĢåÖŹĮæ=ÓŅÅĢķĄĀėÖŹĮæ+ÓĪĀėÖŹĮ棻
£Ø4£©ÅäÖĘ×īŗóŠčµßµ¹Ņ”ŌČ£¬Ź¹ÓĆČŻĮæĘæŹ¹ÓĆĒ°±ŲŠė¼ģ²éŹĒ·ńĀ©Ė®£»
£Ø5£©øł¾Żc=$\frac{n}{V}$ÅŠ¶ĻÅäÖĘČÜŅŗµÄÅØ¶ČŹĒ·ńĘ«“ó»ņĘ«Š”£¬Čē¹ūnĘ«“ó»ņvĘ«Š”£¬ÅäÖĘČÜŅŗµÄÅضČĘ«øߣ¬Čē¹ūnĘ«Š”»ņvĘ«“ó£¬ÅäÖĘČÜŅŗµÄÅØ¶Č¾ĶĘ«µĶ£®
½ā“š ½ā£ŗ£Ø1£©ÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗŅ»°ć²½ÖčĪŖ£ŗ¼ĘĖć”¢³ĘĮ攢Čܽā”¢ĄäČ“”¢ŅĘŅŗ”¢Ļ“µÓ”¢¶ØČŻ”¢Ņ”ŌČµČ£¬ĖłŅŌÕżČ·µÄ²Ł×÷Ė³ŠņĪŖ£ŗ¢Ś¢Ł¢Ū¢ą¢Ż¢Ž¢ß¢Ü£»
¹Ź“š°øĪŖ£ŗ¢Ś¢Ł¢Ū¢ą¢Ż¢Ž¢ß¢Ü£»
£Ø2£©ÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗŅ»°ć²½ÖčĪŖ£ŗ¼ĘĖć”¢³ĘĮ攢Čܽā”¢ĄäČ“”¢ŅĘŅŗ”¢Ļ“µÓ”¢¶ØČŻ”¢Ņ”ŌČ£¬ÓƵ½µÄŅĒĘ÷£ŗĶŠÅĢĢģĘ½”¢Ōæ³×”¢ĮæĶ²”¢ÉÕ±”¢²£Į§°ō”¢ČŻĮæĘ攢½ŗĶ·µĪ¹Ü£¬ÅäÖĘ0.5mol/LµÄNaOHČÜŅŗ500mL£¬Ó¦Ń”Ōń500mLČŻĮæĘ棬»¹Č±ÉŁµÄŅĒĘ÷£ŗÉÕ±”¢²£Į§°ō”¢½ŗĶ·µĪ¹Ü£»
¹Ź“š°øĪŖ£ŗ500mL£»ÉÕ±”¢²£Į§°ō”¢½ŗĶ·µĪ¹Ü£»
£Ø3£©ĢģĘ½³ĘĮæĪļĢåŹ±×ńŃ×óĪļÓŅĀėµÄŌŌņ£¬ŌŚøĆŹµŃéĶ¼ÖŠæÉŅŌ擳ö£¬øĆĶ¬Ń§ŌŚ²Ł×÷Ź±µÄŅ»øö“ķĪóŹĒķĄĀėÓėÉÕ±·Å·“ĮĖĪ»ÖĆ£¬øł¾ŻĢģĘ½Ę½ŗāŌĄķ£ŗ×óÅĢĪļĢåÖŹĮæ=ÓŅÅĢķĄĀėÖŹĮæ+ÓĪĀėÖŹĮ棬Čō¹ū·Å·“ĮĖ£¬Ōņ×óÅĢķĄĀėÖŹĮæ=ÓŅÅĢĪļĢåÖŹĮæ+ÓĪĀėÖŹĮ棬ĖłŅŌÓŅÅĢĪļĢåÖŹĮæ=×óÅĢķĄĀėÖŹĮæ-ÓĪĀėÖŹĮæ=30g-2.4g=27.6g£®
¹Ź“š°øĪŖ£ŗķĄĀėÓėÉÕ±·Å·“ĮĖĪ»ÖĆ£»27.6g£»
£Ø4£©ÅäÖĘ×īŗóŠčµßµ¹Ņ”ŌČ£¬Ź¹ÓĆČŻĮæĘæŹ¹ÓĆĒ°±ŲŠė¼ģ²éŹĒ·ńĀ©Ė®£»
¹Ź“š°øĪŖ£ŗ¼ģ²éŹĒ·ńĀ©Ė®£»
£Ø5£©¢ŁĆ»ÓŠĻ“µÓÉÕ±ŗĶ²£Į§°ō£¬µ¼ÖĀČÜÖŹµÄĪļÖŹµÄĮæĘ«µĶ£¬øł¾Żc=$\frac{n}{V}$æÉÖŖÅäÖĘČÜŅŗµÄÅضČĘ«µĶ£¬¹Ź¢Ł“ķĪó£®
¢Ś×ŖŅĘČÜŅŗŹ±²»É÷ÓŠÉŁĮæČ÷µ½ČŻĮæĘæĶāĆę£¬µ¼ÖĀČÜÖŹµÄĪļÖŹµÄĮæĘ«µĶ£¬øł¾Żc=$\frac{n}{V}$æÉÖŖÅäÖĘČÜŅŗµÄÅضČĘ«µĶ£¬¹Ź¢Ś“ķĪó£®
¢ŪČŻĮæĘæ²»øÉŌļ£¬ŗ¬ÓŠÉŁĮæÕōĮóĖ®£¬ČÜÖŹµÄĪļÖŹµÄĮæŗĶČÜŅŗµÄĢå»ż¶¼²»øı䣬øł¾Żc=$\frac{n}{V}$æÉÖŖÅäÖĘČÜŅŗµÄÅضČĪŽÓ°Ļģ£¬¹Ź¢Ū“ķĪó£®
¢Ü¶ØČŻŹ±ø©ŹÓ±źĻߣ¬µ¼ÖĀČÜŅŗµÄĢå»żĘ«Š”£¬øł¾Żc=$\frac{n}{V}$æÉÖŖÅäÖĘČÜŅŗµÄÅضČĘ«øߣ¬¹Ź¢ÜÕżČ·£®
¢Ż³Ę¶ØČŻŹ±ŃöŹÓ±źĻߣ¬µ¼ÖĀČÜŅŗµÄĢå»żĘ«“ó£¬øł¾Żc=$\frac{n}{V}$æÉÖŖÅäÖĘČÜŅŗµÄÅضČĘ«µĶ£¬¹Ź¢Ż“ķĪó£®
¢Ž¶ØČŻŗóČūÉĻĘæČū·“ø“Ņ”ŌČ£¬¾²ÖĆŗó£¬ŅŗĆęµĶÓŚæĢ¶ČĻߣ¬ŌŁ¼ÓĖ®ÖĮæĢ¶ČĻߣ¬µ¼ÖĀČÜŅŗµÄĢå»żĘ«“ó£¬øł¾Żc=æÉÖŖÅäÖĘČÜŅŗµÄÅضČĘ«µĶ£¬¹Ź¢Ž“ķĪó£®
¹ŹŃ”£ŗ¢Ü£®
µćĘĄ ±¾Ģāæ¼²éĮĖŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗµÄÅäÖĘøł¾ŻŗĶ×¢ŅāŹĀĻī£¬Ć÷Č·ÅäÖĘŌĄķ¼°ČŻĮæĘ攢ĶŠÅĢĢģĘ½Ź¹ÓĆ·½·ØŗĶ×¢ŅāŹĀĻīŹĒ½āĢā¹Ų¼ü£¬ĢāÄæÄŃ¶Č²»“ó£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¼×Č©ČÜŅŗ | B£® | ¾Ę¾« | C£® | ±„ŗĶ£ØNH4£©2SO4ČÜŅŗ | D£® | “×ĖįĒ¦ČÜŅŗ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
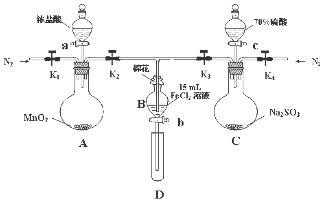 ĪŖĮĖŃéÖ¤Ńõ»ÆŠŌCl2£¾Fe3+£¾SO2£¬Ä³Š”×éÓĆČēĶ¼ĖłŹ¾×°ÖĆ½ųŠŠŹµŃé£Ø¼Š³ÖŅĒĘ÷ŗĶAÖŠ¼ÓČČ×°ÖĆŅŃĀŌ£¬ĘųĆÜŠŌŅŃ¼ģŃ飩£®
ĪŖĮĖŃéÖ¤Ńõ»ÆŠŌCl2£¾Fe3+£¾SO2£¬Ä³Š”×éÓĆČēĶ¼ĖłŹ¾×°ÖĆ½ųŠŠŹµŃé£Ø¼Š³ÖŅĒĘ÷ŗĶAÖŠ¼ÓČČ×°ÖĆŅŃĀŌ£¬ĘųĆÜŠŌŅŃ¼ģŃ飩£®| ¹ż³Ģ¢ōBČÜŅŗÖŠŗ¬ÓŠµÄĄė×Ó | ¹ż³Ģ¢öBČÜŅŗÖŠŗ¬ÓŠµÄĄė×Ó | |
| ¼× | ÓŠFe3+ĪŽFe2+ | ÓŠSO42- |
| ŅŅ | ¼ČÓŠFe3+ÓÖÓŠFe2+ | ÓŠSO42- |
| ±ū | ÓŠFe3+ĪŽFe2+ | ÓŠFe2+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | »Æѧ·“Ó¦°éĖęÄÜĮæ±ä»Æ£¬ŹĒ»Æѧ·“Ó¦µÄ»ł±¾ĢŲÕ÷Ö®Ņ» | |
| B£® | ³£ĪĀĻĀ£¬ĒāŃõ»Æ±µ¾§ĢåÓėĀČ»Æļ§¾§Ģå»ģŗĻ·Å³ö°±Ęų£¬øĆ·“Ó¦ĪŖ·ÅČČ·“Ó¦ | |
| C£® | »Æѧ·“Ó¦ÖŠÄÜĮæ±ä»ÆµÄ“óŠ”Óė·“Ó¦ĪļµÄÖŹĮæ¶ąÉŁĪŽ¹Ų | |
| D£® | ¾É»Æѧ¼ü¶ĻĮŃĖł·Å³öµÄÄÜĮæøßÓŚŠĀ»Æѧ¼üŠĪ³ÉĖłĪüŹÕµÄÄÜĮæŹ±·¢Éś·ÅČČ·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 68.76 kJ | B£® | 57.3 kJ | C£® | 34.38 kJ | D£® | 17.19 kJ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
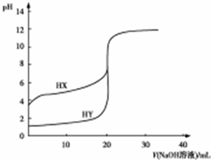 ³£ĪĀĻĀ£¬ĻÖÓŠÅØ¶Č¶¼ĪŖ0.1mol•L-1HX”¢HYµÄČÜŅŗø÷20ml£¬·Ö±šÓĆ0.1mol•L-1NaOHČÜŅŗµĪ¶Ø£®ČÜŅŗµÄpHÓė¼ÓČėNaOHČÜŅŗĢå»żVµÄ¹ŲĻµČēĶ¼ĖłŹ¾£®
³£ĪĀĻĀ£¬ĻÖÓŠÅØ¶Č¶¼ĪŖ0.1mol•L-1HX”¢HYµÄČÜŅŗø÷20ml£¬·Ö±šÓĆ0.1mol•L-1NaOHČÜŅŗµĪ¶Ø£®ČÜŅŗµÄpHÓė¼ÓČėNaOHČÜŅŗĢå»żVµÄ¹ŲĻµČēĶ¼ĖłŹ¾£®| A£® | µĪ¶Ø¹ż³ĢÖŠ£¬Ė®µēĄėµÄc£ØH+£©•c£ØOH-£©¾ł²»±ä | |
| B£® | HXµÄµēĄė³Ģ¶ČŠ”ÓŚHYµÄµēĄė³Ģ¶Č | |
| C£® | V=10mlŹ±£¬c£ØHX£©+c£ØX-£©=2c£ØNa+£© | |
| D£® | V=20mlŹ±£¬c£ØY-£©£¾c£ØX-£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | I2 | B£® | K2SO4 | C£® | P2O5 | D£® | HNO3 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com