


 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A����̿���½����ڿ����е�ȼ�����Եõ�ʹ����ʯ��ˮ����ǵ����� |
| B����̿���½����гɷ�ĩ�����뵽ϡ�ĺ�īˮ�У���īˮ��ɫ |
| C����̿���½����гɷ�ĩ��������ͭ��ĩ��ϼ��ȣ����Եõ���ɫ���� |
| D����̿���½��ϼ��뵽ʢ�������ļ���ƿ�У�����һ��ʱ�䣬����ɫ��dz����ԭ����̿���½����е���������������������ԭ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Cl2 | B��Br2 | C��HCl | D��CO2 |
�鿴�𰸺ͽ���>>
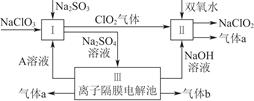
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
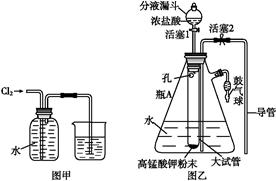
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
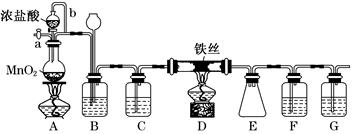
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
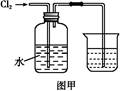
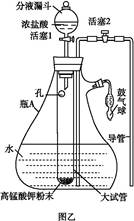
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
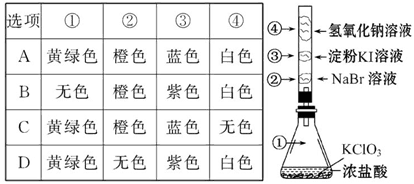
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������£�Ũ����������������棬˵������Ũ�����Ӧ |
| B��Ũ�����ڹ��յ���������ɫ��ƣ�˵��Ũ����ȶ� |
| C���������м���Ũ�������ַ�������˵��Ũ���������ˮ�� |
| D����ӦCuSO4��H2S=CuS����H2SO4�ܽ��У�˵����ͭ�Ȳ�����ˮ��Ҳ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ڱ�����ˮ�м���CaCO3����Һ��pH��� |
| B���ڱ�����ˮ��ͨ��SO2���壬��Һ��pH��С |
| C���ڱ�����ˮ��ͨ��H2S���壬��Һ��pH��С |
| D���ڱ�����ˮ�м���NaOHʹpH��7��������Һ������Ũ�ȵĹ�ϵ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com