
����Ŀ���±�ΪԪ�����ڱ���ǰ�����ڵIJ���Ԫ�أ��������е���ĸ�ֱ����һ�ֻ�ѧԪ�أ�����Ҫ��ش����и�С�⣺
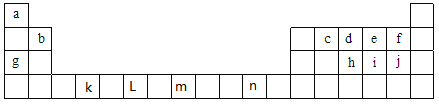
��1����Ԫ�طǽ�����ǿ���Ƚ��кܶ��������f��j�ķǽ�����ǿ�����о������в����е���_________������ţ�
a.�Ƚ����ֵ��ʵ���ɫ b.�Ƚ��⻯����ȶ��� c.������Ԫ�������ڱ���λ��
d.�Ƚϵ縺�� e.�Ƚ�����������Ӧˮ���������
�ڸ���Ԫ��ԭ�ӵ���Χ�����Ų����������ɽ�Ԫ�����ڱ�ǰ������Ԫ�طֳ�4�����ֱ�Ϊs����p����d����ds����������s����Ԫ����_______�֣�����d����Ԫ����_______�֣�Ԫ��n����________����
����c��d��e����Ԫ���У��縺����С�����˳����______________,��һ�������ɴ�С��˳����____________(��Ԫ�ط��Żش�)��
��2����д�� n2���ĺ�������Ų�ʽ��______________________��
��д��kԪ�ػ�̬ԭ�ӵļ۵����Ų�ʽ��_____________��
��д�� LԪ�ػ�̬ԭ�ӵ���Χ�����Ų�ʽ��_____________��
��д��mԪ�ػ�̬ԭ���۵����Ĺ����ʾʽ��________________________����Ԫ����Ԫ�����ڱ��е�λ��Ϊ��__________________________��
��j�������ӵĽṹʾ��ͼΪ____________��
��3����Ԫ��i���⻯���������____________������ԡ��Ǽ��ԡ������ӣ�����ӵĿռ乹��Ϊ____________�����⻯�������iԭ�ӹ�����ӻ�������__________�� ��i��e�γɵ�ie42�����ӣ���ռ乹��Ϊ__________(����������)��
����֪cd- �� d2 �ṹ���ƣ�1 mol cd- ��![]() ����ĿΪ___________����d�γɵ�����d3����CO2��Ϊ�ȵ����壬��d3���ķ��ӹ���Ϊ___________��
����ĿΪ___________����d�γɵ�����d3����CO2��Ϊ�ȵ����壬��d3���ķ��ӹ���Ϊ___________��
��f2ͨ��ϡNaOH��Һ�п�����Of2,Of2���ӹ���Ϊ___________��������ԭ�ӵ��ӻ���ʽΪ_______��
�ܻ�����j2e�����幹��Ϊ_________������ԭ�ӵļ۲���Ӷ���Ϊ__________��
���𰸡� ae 7�� 8�� ds C��N��O N>O>C 1s22s22p63s23p63d9��[Ar]3d9 3d24s2 3d54s1 ![]() ��4���ڵ�VIII��
��4���ڵ�VIII�� ![]() ���� V�� sp3 �������� 2NA ֱ���� V�� sp3 V�� 4
���� V�� sp3 �������� 2NA ֱ���� V�� sp3 V�� 4
������������Ԫ�������ڱ��е�λ�ÿ�֪��aΪHԪ�ء�bΪBeԪ�ء�cΪCԪ�ء�dΪNԪ�ء�eΪOԪ�ء�fΪFԪ�ء�gΪNaԪ�ء�hΪPԪ�ء�iΪSԪ�ء�jΪClԪ����kΪTiԪ�ء�LΪCrԪ�ء�mΪFeԪ����nΪCuԪ�ء�
(1). ��. a.��ɫ�����������ʣ����ܱȽϷǽ�����ǿ������a����b.�ǽ�����Խǿ����̬�⻯��Խ�ȶ������ԱȽϷǽ�����ǿ������b��ȷ��c.fΪFԪ�ء�jΪClԪ�أ�ͬһ����Ԫ��������ԭ����������Ԫ�صķǽ����Լ��������ԱȽϷǽ�����ǿ������c��ȷ��d.�ǽ�����Խǿ���縺��Խ���ԱȽϷǽ�����ǿ������d��ȷ��e.fΪFԪ�أ�������������Ӧ��ˮ������ܱȽϷǽ�����ǿ������e����ѡ��ae��
��.s������IA���IIA�壬ǰ����������s����Ԫ������H��Li��Be��Na��Mg��K��Ca����7����d������IIIB~VIIB���VIII��(��ϵԪ�غ��ϵԪ�س���)��ǰ����������d����Ԫ������Sc��Ti��V��Cr��Mn��Fe��Co��Ni����8����n��CuԪ�أ�λ��IB�壬����ds��Ԫ�أ��ʴ�Ϊ��7����8����ds��
��.Ԫ�صķǽ�����Խǿ����縺��Խ����C��N��O����Ԫ���У��ǽ�����C��N��O�����Ե縺��C��N��O��ͬһ���ڵ�Ԫ�أ�����ԭ������������һ�����ܳ��������ƣ���NԪ�ص�2p�ܼ�Ϊ��������ȶ��ṹ�����Ե�һ������N>O>C���ʴ�Ϊ��C��N��O��N>O>C��
(2). ��.nΪCuԪ�أ�Cu��29��Ԫ�أ���Cu2+�ĺ�������Ų�ʽΪ1s22s22p63s23p63d9��[Ar]3d9���ʴ�Ϊ��1s22s22p63s23p63d9��[Ar]3d9��
��.kΪ22��TiԪ�أ����̬ԭ�ӵļ۵����Ų�ʽΪ3d24s2���ʴ�Ϊ��3d24s2��
��.LΪ24��CrԪ�أ����̬��������Ų�ʽΪ1s22s22p63s23p63d54s1����CrԪ�صĻ�̬ԭ�ӵ���Χ�����Ų�ʽΪ3d54s1���ʴ�Ϊ��3d54s1��
��.mΪ26��FeԪ�أ����̬��������Ų�ʽΪ1s22s22p63s23p63d64s2�����̬ԭ�ӵļ۵��ӹ����ʾʽΪ![]() ��FeԪ��λ��Ԫ�����ڱ��ĵ�4���ڵ�VIII�壬�ʴ�Ϊ��
��FeԪ��λ��Ԫ�����ڱ��ĵ�4���ڵ�VIII�壬�ʴ�Ϊ��![]() ����4���ڵڢ��壻
����4���ڵڢ��壻
��.jΪClԪ�أ�Cl���Ľṹʾ��ͼΪ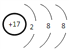 ���ʴ�Ϊ��
���ʴ�Ϊ��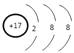 ��
��
(3). ��.H2S���ӵ�����ԭ�Ӽ۲���Ӷ���Ϊ2+![]() =2+2=4����Sԭ�ӵ��ӻ�����Ϊsp3���µ��Ӷ���Ϊ2������H2S���ӵĿռ乹��ΪV
=2+2=4����Sԭ�ӵ��ӻ�����Ϊsp3���µ��Ӷ���Ϊ2������H2S���ӵĿռ乹��ΪV![]() =4+0=4���µ��Ӷ���Ϊ0����SO42���Ŀռ乹��Ϊ�������壬�ʴ�Ϊ�����ԣ�V�Σ�sp3���������壻
=4+0=4���µ��Ӷ���Ϊ0����SO42���Ŀռ乹��Ϊ�������壬�ʴ�Ϊ�����ԣ�V�Σ�sp3���������壻
��. 1��N2�����к���1���Ҽ���2���м���CN����N2�Ľṹ���ƣ���1mol CN���Цм���ĿΪ2NA��CO2��ֱ���η��ӣ�N3����CO2��Ϊ�ȵ����壬��N3���ķ��ӹ���Ϊֱ���Σ��ʴ�Ϊ��2NA��ֱ���Σ�
��.F2ͨ��ϡNaOH��Һ�п�����OF2��OF2���ӵ�����ԭ�Ӽ۲���Ӷ���Ϊ2+![]() =2+2=4���µ��Ӷ���Ϊ2��������ӿռ乹��ΪV�Σ���ԭ�ӵ��ӻ���ʽΪsp3���ʴ�Ϊ��V�Σ�sp3��
=2+2=4���µ��Ӷ���Ϊ2��������ӿռ乹��ΪV�Σ���ԭ�ӵ��ӻ���ʽΪsp3���ʴ�Ϊ��V�Σ�sp3��
��.������Cl2O������ԭ�Ӽ۲����Ϊ2+![]() =2+2=4���µ��Ӷ���Ϊ2���������幹��ΪV�����ʴ�Ϊ��V�Σ�4��
=2+2=4���µ��Ӷ���Ϊ2���������幹��ΪV�����ʴ�Ϊ��V�Σ�4��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪Һ���ܺ�NaH��Ӧ�ų�H2��NaH + NH3 =NaNH2 + H2������Ҳ�ܺ�Na��Ӧ�ų�H2���ݴ�����˵���д�������� ��
A. Һ����NaH��Ӧ�У�Һ����������
B. Һ����NaH��Ӧ���ɵ�H2���������������ǻ�ԭ����
C. Һ����Na��Ӧ��������NaNH2
D. Һ����NaH��Na��Ӧ�������û���Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и��������У����ǹ��ۻ�������ǣ� ��
A��H2S��Na2O2 B��H2O2��CaF2 C��NH3��N2 D��HNO3��HClO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������һ�ֳ�Ӳ��ĥͿ����ϣ���ͼΪ�侧�������е�ÿ��ԭ�Ӿ�����8�����ȶ��ṹ�������й�˵����ȷ����( )

A. ��������۵�ܵ�
B. ������Ļ�ѧʽΪBP���������Ӿ���
C. ��������ÿ��ԭ�Ӿ��γ�4�����ۼ�
D. ������ṹ�����Ŀռ�ѻ���ʽ���Ȼ�����ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ԭ�ӽṹ��Ԫ�����ڱ���Ԫ���������߹�ϵ���С�
A��B��D��E��FΪԭ���������������ǰ������Ԫ�أ�����A�����������������ڲ��������2����B��D��EΪͬ����Ԫ�أ�Bԭ�ӵĺ��������������δ�ɶԵ�������5����Eԭ���������1��δ�ɶԵ��ӣ�Fԭ�Ӻ�����22���˶�״̬�ĵ��ӡ�
��ش��������⣺
��1��FԪ��λ�����ڱ�_____________������۵����Ų�ͼΪ��_____________��
��2��B��D��E����Ԫ���У���һ��������С����_____________ (��Ԫ�ط���)��д��AD2�ĵȵ�����_____________ (���Ӻ������Ӹ�дһ��)��
��3��AO2��DO2�۵�ߵ���_____________��ԭ����_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���о�����Ľṹ�Ի�ѧ�²��ϵķ���������Ҫ�ļ�ֵ��
��1����������һ��ԭ�ӵ�����λ�þ����ó�֮Ϊԭ�ӷ�������������ֱ�С��1������x��y��z�������Թ涨��ij����ľ����ṹ��ͼ��ʾ��1��ԭ������Ϊ��0��0��0����2��ԭ������Ϊ��1/3��2/3��0�����þ���Ļ�ѧʽΪ___________���þ�������Ϊ��a = 250.4 pm, c = 666.1 pm���� = 120o�� 3��ԭ������Ϊ_____________���г�����������A��B��ԭ�Ӽ����С�˼��ļ���ʽΪ_____________�������������ֵ������Ҫ����

��2�����������ѻ��ľ����Ǹ������壬����뾶Ϊr��ԭ�ӱ��ֽ��ܽӴ�����������������ݵ��°뾶���Ϊ___________��һ��ԭ�ӡ�
��3��Fe���γɶ������������FeO�����ṹΪNaCl�͡�������ʵ���ϴ��ڿ�λ����λ������ԭ�ӵ�ȱ�ݣ�����ȱ�ݶԾ�������ʻ�����ش�Ӱ�졣���ھ���ȱ�ݣ��ھ�����Fe��O�ĸ����ȷ����˱仯����ΪFexO(x��1)�������ijFexO�����ܶ�Ϊ5.71 g��cm��3�������߳�Ϊ4.28��10��10 m����FexO��x��__________(���������λ��Ч����)��
��4�����ѿ���Ľṹ��ͼ��ʾ������������ӿ���Ӳ��Ӵ�ģ�ͣ������Ӻ���������������ӵĿ�϶���������γ��������壬������λ�������������ģ���һ�������ӱ�__________�������Ӱ�Χ��������λ���������������ģ�һ�������ӱ�_____�������Ӱ�Χ�����ѿ���Ļ�ѧʽΪ__________���������Ӱ뾶Ϊa pm������ѿ��������������Ӽ���̾���Ϊ_______pm��������������Ӽ���̾���Ϊ_______pm��

��5����Ԫ��������ͬ�������壬�������������ѻ��������������������ѻ�����ͼ��ʾF����Ľṹ�У���������a=0.295nm��c=0.469nm�����F������ܶ�Ϊ_____________ g�� cm-3
(��NA��ʾ�����ӵ�������ֵ���г�����ʽ���ɣ����û���)��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й�����������������ȷ���ǣ� ��
A. ����ֻ����̼��������Ԫ��
B. ����̼����Ԫ�ص�����һ��������
C. ����һ���DZ���������������һ��������
D. ̼ԭ�Ӽ�ֻ�Ե������ϵ���һ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��ʱ��Fe(OH)2��Cu(OH)2�ı�����Һ�У����������ӵ����ʵ���Ũ�ȵĸ�����[-lgc(M2+)]����ҺpH�ı仯��ϵ��ͼ��ʾ����֪���¶���Ksp[Cu(OH)2]<Ksp[Fe(OH)2]������˵����ȷ����
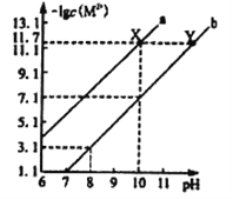
A. ����a��ʾFe(OH)2������Һ�еı仯��ϵ
B. ��ȥCuSO4��Һ�к��е�����Fe2+���ɼ�����CuO
C. ��Fe(OH)2��Cu(OH)2��������ʱ����Һ��c(Fe2+)��c(Cu2+)=104.6 ��1
D. ��X���Ӧ�ı�����Һ�м�������NaOH���壬��ת��ΪY���Ӧ����Һ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com