
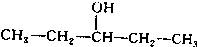 £®
£®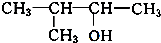 ”¢
”¢ Š“³öĮķĶāĮ½ÖÖĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½£ŗ
Š“³öĮķĶāĮ½ÖÖĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½£ŗ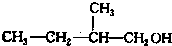 ”¢
”¢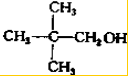 £®
£® ·ÖĪö £Ø1£©øł¾ŻĪļÖŹ¹ŁÄÜĶÅĄ“ÅŠ¶Ļ£»¹ŁÄÜĶÅŅģ¹¹ŹĒÖøŗ¬ŅåµÄ¹ŁÄÜĶŲ»Ņ»ŃłµÄĶ¬·ÖŅģ¹¹Ģ壻Ģ¼Į“Ņģ¹¹ŹĒÖøŗ¬ÓŠµÄ¹ŁÄÜĶÅŅ»Ńł£¬µ«ŹĒĢ¼Į“²»Ņ»ŃłµÄĶ¬·ÖŅģ¹¹ĢåµÄÓŠ»śĪļ£»¹ŁÄÜĶÅĪ»ÖĆŅģ¹¹ŹĒÖø¹ŁÄÜĶŵÄĪ»ÖĆ²»Ķ¬£¬µ«ŹĒ¹ŁÄÜĶÅĻąĶ¬µÄŅģ¹¹Ģ壻
£Ø2£©»„ĪŖ¹ŁÄÜĶÅĪ»ÖĆŅģ¹¹µÄÓŠ»śĪļÖ»ŅŖ½«¹ŁÄÜĶŵÄĪ»ÖƱä»Æ¼“æɵƵ½£»
£Ø3£©øł¾ŻĢ¼Į“Ņģ¹¹µÄŹéŠ“·½·ØĄ“»Ų“š£¬×¢ŅāĪģ»łµÄĢ¼Į“Ņģ¹¹µÄŹéŠ“·½·Ø£®
½ā“š ½ā£ŗ£Ø1£©AÖŠŗ¬ÓŠ“¼ōĒ»ł£¬ŹōÓŚ“¼Ąą£¬BÓėA»„ĪŖ¹ŁÄÜĶÅŅģ¹¹µÄŹĒ£¬DÓėA»„ĪŖĢ¼Į“Ņģ¹¹£¬CÓėA»„ĪŖ¹ŁÄÜĶÅĪ»ÖĆŅģ¹¹£¬¹Ź“š°øĪŖ£ŗ“¼£»B£»D£»C£»
£Ø2£©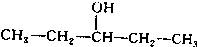 ÓėA»„ĪŖ¹ŁÄÜĶÅĪ»ÖĆŅģ¹¹£¬¹Ź“š°øĪŖ£ŗ
ÓėA»„ĪŖ¹ŁÄÜĶÅĪ»ÖĆŅģ¹¹£¬¹Ź“š°øĪŖ£ŗ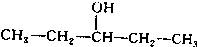 £»
£»
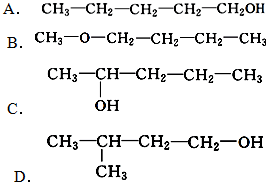
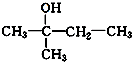
£Ø3£©ÓėA»„ĪŖĢ¼Į“Ņģ¹¹µÄĶ¬·ÖŅģ¹¹Ģ壬æÉŅŌøł¾ŻĪģ»łµÄĢ¼Į“Ņģ¹¹Ą“ŹéŠ“£¬Īģ»łÓŠCH3CH2CH2CH2CH2-£¬CH3CH2CH2CH£ØCH3£©-£¬CH3CH2CH£ØCH2CH3£©-£¬£ØCH3£©2CHCH2CH2-£¬£ØCH3£©3CCH2-£¬ĖłŅŌĮķĶāĮ½ÖÖĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½ĪŖ£ŗ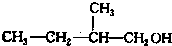 ”¢
”¢ £¬¹Ź“š°øĪŖ£ŗ
£¬¹Ź“š°øĪŖ£ŗ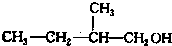 ”¢
”¢ £®
£®
µćĘĄ ±¾Ģāæ¼²éѧɜĶ¬·ÖŅģ¹¹ĢåµÄĄą±šŅŌ¼°Ņģ¹¹ĢåµÄŹéŠ“µČÖŖŹ¶£¬×¢ŅāÖŖŹ¶µÄ¹éÄÉŗĶŹįĄķŹĒ¹Ų¼ü£¬ÄѶČÖŠµČ£®


 ĄųŌÅŹéŅµŹī¼ŁĻĪ½ÓÄž²Ø³ö°ęÉēĻµĮŠ“š°ø
ĄųŌÅŹéŅµŹī¼ŁĻĪ½ÓÄž²Ø³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

| ŹµŃéŠņŗÅ | ĘšŹ¼ĪĀ¶Čt1/”ę | ÖÕÖ¹ĪĀ¶Č£Øt2£©”ę | ĪĀ²ī£Øt2-t1£©”ę | ||
| ŃĪĖį | NaOHČÜŅŗ | Ę½¾łÖµ | |||
| 1 | 25.1 | 24.9 | 25.0 | 31.6 | 6.6 |
| 2 | 25.1 | 25.1 | 25.1 | 31.8 | 6.7 |
| 3 | 25.1 | 25.1 | 25.1 | 31.9 | 6.8 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā

| A£® | 4£ŗ1 | B£® | 1£ŗ2 | C£® | 2£ŗ1 | D£® | 1£ŗ1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
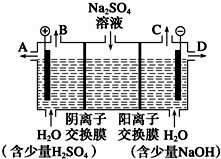 ĢģČ»æóĪļĆ¢Ļõ»ÆѧŹ½ĪŖNa2SO4•10H2O£¬ĪŖĪŽÉ«¾§Ģ壬Ņ×ČÜÓŚĖ®£®øĆŠ”×éĶ¬Ń§ÉčĻė£¬Čē¹ūÄ£Äā¹¤ŅµÉĻĄė×Ó½»»»Ä¤·ØÖĘÉÕ¼īµÄ·½·Ø£¬ÓĆČēĶ¼ĖłŹ¾×°ÖƵē½āĮņĖįÄĘČÜŅŗĄ“ÖĘČ”ĒāĘų”¢ŃõĘų”¢ĮņĖįŗĶĒāŃõ»ÆÄĘ£¬ĪŽĀŪ“Ó½ŚŹ”ÄÜŌ“»¹ŹĒ“ÓĢįøßŌĮĻµÄĄūÓĆĀŹ¶ųŃŌ¶¼øü¼Ó·ūŗĻĀĢÉ«»ÆѧĄķÄī£®
ĢģČ»æóĪļĆ¢Ļõ»ÆѧŹ½ĪŖNa2SO4•10H2O£¬ĪŖĪŽÉ«¾§Ģ壬Ņ×ČÜÓŚĖ®£®øĆŠ”×éĶ¬Ń§ÉčĻė£¬Čē¹ūÄ£Äā¹¤ŅµÉĻĄė×Ó½»»»Ä¤·ØÖĘÉÕ¼īµÄ·½·Ø£¬ÓĆČēĶ¼ĖłŹ¾×°ÖƵē½āĮņĖįÄĘČÜŅŗĄ“ÖĘČ”ĒāĘų”¢ŃõĘų”¢ĮņĖįŗĶĒāŃõ»ÆÄĘ£¬ĪŽĀŪ“Ó½ŚŹ”ÄÜŌ“»¹ŹĒ“ÓĢįøßŌĮĻµÄĄūÓĆĀŹ¶ųŃŌ¶¼øü¼Ó·ūŗĻĀĢÉ«»ÆѧĄķÄī£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
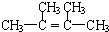 £»
£»²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| ŃōĄė×Ó | Na+”¢K+”¢Cu2+ |
| ŅõĄė×Ó | SO42-”¢OH- |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
 µēĘæ³µĖłÓƵē³ŲŅ»°ćĪŖĒ¦Šīµē³Ų£¬ÕāŹĒŅ»ÖÖµäŠĶµÄæɳäµēµē³Ų£¬µē³Ų×Ü·“Ó¦Ź½ĪŖ£ŗ2PbSO4+2H2O $?_{·Åµē}^{³äµē}$Pb+PbO2+4H++2SO42-£®ŌņĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©
µēĘæ³µĖłÓƵē³ŲŅ»°ćĪŖĒ¦Šīµē³Ų£¬ÕāŹĒŅ»ÖÖµäŠĶµÄæɳäµēµē³Ų£¬µē³Ų×Ü·“Ó¦Ź½ĪŖ£ŗ2PbSO4+2H2O $?_{·Åµē}^{³äµē}$Pb+PbO2+4H++2SO42-£®ŌņĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©| A£® | ·ÅµēŹ±£ŗµē×Ó·½ĻņÓÉBµ½A | |
| B£® | ·ÅµēŹ±£ŗÕż¼«·“Ó¦ŹĒ Pb-2e-+SO42-ØTPbSO4 | |
| C£® | ³äµēŹ±£ŗŃō¼«·“Ó¦ŹĒPbSO4+2H2O-2e-ØTPbO2+SO42-+4H+ | |
| D£® | ³äµēŹ±£ŗĒ¦Šīµē³ŲµÄøŗ¼«Ó¦Óė³äµēĘ÷µēŌ“µÄÕż¼«ĻąĮ¬ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ģ¼ĖįøĘÓėĻ”ŃĪĖį»ģŗĻ£ŗCaCO3+2H+ØTCa2++CO2”ü+H2O | |
| B£® | ĮņĖįĶČÜŅŗÓėÉÕ¼īČÜŅŗ»ģŗĻ£ŗCu2++2OH-ØTCu£ØOH£©2”ż | |
| C£® | Ģ¼ĖįĒāÄĘÓėĻ”ŃĪĖį»ģŗĻ£ŗHCO3-+H+ØTCO2”ü+H2O | |
| D£® | °Ń¶žŃõ»ÆĢ¼ĶØČėĀČ»ÆøĘČÜŅŗÖŠ£ŗCa2++H2O+CO2ØTCaCO3”ż+2H+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | NH3 | B£® | CH4 | C£® | NaHCO3 | D£® | HNO3 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com