
| A�� | �����ʾ��Һ�У�����Ϊδ��Ӧ���CH3COONa�����ɵ�CH3COOH��NaCl����֮��Ĺ�ϵΪ��c��CH3COOH����c��Cl-����c��OH-��=c��H+�� | |
| B�� | �����ʾ��Һ�У���������һ�������ᷴӦ����֮��Ĺ�ϵΪ��c��Na+����c��Cl-����c��CH3COO-����c��CH3COOH�� | |
| C�� | �����ʾ��Һ�У�������������ǡ����ȫ��Ӧ����֮��Ĺ�ϵΪ��c��Na+����c��CH3COOH����c��H+����c��CH3COO-�� | |
| D�� | �����������п��ܳ��֣�c��H+��+c��Na+��=c��CH3COOH��+c��CH3COO-�� |
���� A��CH3COONa��HClǡ�÷�Ӧ�����ԣ���֪�����ԣ�˵��CH3COONa�����������Һ�ĵ���غ㡢�����غ�����жϸ�����Ũ�ȴ�С��
B�����ʱ����Һ�д���CH3COONa��CH3COOH����Һ�����ԣ�Ӧ��c��CH3COO-����c��Cl-����
C�����ʱ��ǡ�÷�Ӧ����CH3COOH��CH3COOHΪ������ʣ�����ȫ���룬��Һ�����ԣ�
D������������������Һ��ѭ�����غ�����жϣ�
��� �⣺A��CH3COONa��HClǡ�÷�Ӧ�����ԣ���֪�����ԣ�˵��CH3COONa����������Ϊδ��Ӧ���CH3COONa�����ɵ�CH3COOH��NaCl����Һ���ڵ���غ㣺c��H+��+c��Na+��=c��OH-��+c��CH3COO-��+c��Cl-���������غ㣺c��Na+��=c��CH3COOH��+c��CH3COO-���������ҺpH=7��c��H+��=c��OH-����c��Na+��=c��CH3COO-��+c��Cl-�����������Ϲ�ϵ�ɵ�c��CH3COOH��=c��Cl-��������Һ������Ũ�ȴ�С��ϵΪ��c��CH3COOH��=c��Cl-����c��OH-��=c��H+������A����
B�����ʱ����������10mL����Һ�д���CH3COONa��CH3COOH����Һ�����ԣ�˵��CH3COOH����̶ȴ���CH3COO-ˮ��̶ȣ�Ӧ��c��CH3COO-����c��Cl-�������������غ㣺c��Na+��=c��CH3COOH��+c��CH3COO-���ɵ�c��Na+����c��CH3COO-��������Һ������Ũ�ȴ�С��ϵΪ��c��Na+����c��CH3COO-����c��Cl-����c��CH3COOH������B����
C�����ʱ��ǡ�÷�Ӧ����CH3COOH��CH3COOHΪ������ʣ����Ჿ�ֵ��룬��Һ�����ԣ���c��Na+����c��CH3COOH����c��H+����c��CH3COO-������C��ȷ��
D�����������У���Һ���������غ㣺c��Na+��=c��CH3COOH��+c��CH3COO-������D����
��ѡC��
���� ���⿼��������ϵĶ����жϼ���ҺpH�ļ��㡢��Һ������Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ�ע��������Һ���������ҺpH�Ĺ�ϵ��Ҫ��ѧ���ܹ����ݵ���غ㡢�����غ㡢�ε�ˮ��ԭ���ж���Һ������Ũ�ȴ�С��


 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Cu | B�� | NaOH��Һ | C�� | ����Na2CO3 | D�� | �Ҵ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na+��Cu2+��Cl-��NO3- | B�� | Na+��K+��SO32-��CO32- | ||
| C�� | K+��HCO3-��NO3-��Cl- | D�� | Ca2+��Mg2+��NO3-��Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Fe��OH��3��Ӧ��Fe��OH��3+3H+�TFe3++3H2O | |
| B�� | ϡ���������۷�Ӧ��2Fe+6H+�T2Fe3++3H2�� | |
| C�� | ����������Һ��ϡ���ᷴӦ��Ba2++SO42-�TBaSO4�� | |
| D�� | ̼��������ᷴӦ��CO32-+2H+�TH2O+CO2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ���᳧�����ջ�����FeS2������ȡ���ᣬʵ�����������᳧��������Ҫ�ɷ���Fe2O3������FeS��SiO2���Ʊ��̷���
���᳧�����ջ�����FeS2������ȡ���ᣬʵ�����������᳧��������Ҫ�ɷ���Fe2O3������FeS��SiO2���Ʊ��̷���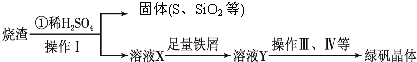
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
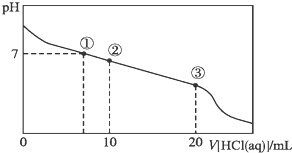
 ��ӦA��g��+B��g��?C��g��+D��g�������е������仯��ͼ��ʾ���ش���������
��ӦA��g��+B��g��?C��g��+D��g�������е������仯��ͼ��ʾ���ش����������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
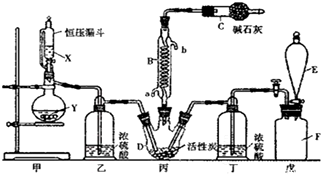
�鿴�𰸺ͽ���>>
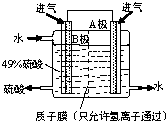
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 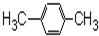 | B�� | 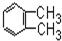 | C�� | 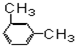 | D�� | 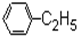 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com