
| A�� | �٢� | B�� | �ڢ� | C�� | �ڢۢ� | D�� | �٢ڢۢ� |
���� ����Ͳ�е�����ˮϡ����Ũ���ᣬ����Ũ����Ũ�ȼ�С��
�ڶ���ʱ��������ƿ�̶��ߣ����¼��������ˮ���ƫС��
�۵ζ����¶˼��촦������һ��Һ�Σ��������ĵı�Һ�����ƫ��
������ˮ��Ӱ�����Һ�����ʵ����ʵ������Եζ����û��Ӱ�죮
��� �⣺������Ͳ��ȡŨ��������0.01mol/Lϡ����ʱ����Ͳ������ˮϴ����δ������ֱ����ȡŨ���ᣬ�ᵼ��Ũ���ᱻ����ˮϡ�ͣ���ҺŨ����Ũ�ȼ�С���ζ�ʱ���ĵı�Һ���������c�����⣩=$\frac{c��������V������}{V��������⣩}$���ⶨ���ƫ�ߣ��ʢ���ȷ��
������ϡ���ᶨ��ʱ����������ƿ�̶��ߣ���������ƿ�м��������ˮ���ƫС�����Ƶ���ҺŨ��ƫ�ߣ��ζ�ʱ���ĵı�Һ���ƫС������c�����⣩=$\frac{c��������V������}{V��������⣩}$���ⶨ���ƫ�ͣ��ʢڴ���
�۵ζ�����ʱ���������ֵζ����¶˼��촦������һ��Һ�Σ��������ĵı�Һ���ƫ����c�����⣩=$\frac{c��������V������}{V��������⣩}$���ⶨ���ƫ�ߣ��ʢ���ȷ��
�ܵζ�����������������ˮ����ƿ�ڱ�ճ����������£��Դ���Һ���ʵ���û��Ӱ�죬���Բ�Ӱ�����ĵı�Һ�����������c�����⣩=$\frac{c��������V������}{V��������⣩}$���Բⶨ����������Ӱ�죬�ʢܴ���
��ѡA��
���� ���⿼�����к͵ζ��е�����������Ŀ�Ѷ��еȣ�ע�������к͵ζ����������ķ��������ɣ������к͵ζ������в��������ʱ��Ҫ����ʵ�������c�����⣩=$\frac{c��������V������}{V��������⣩}$��Ӱ������жϣ�����������ѧ�������Ӧ����ѧ֪ʶ���ʵ�������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| Ԫ�ش��� | L | M | Q | R | T |
| ԭ�Ӱ뾶/nm | 0.160 | 0.143 | 0.089 | 0.102 | 0.074 |
| ��Ҫ���ϼ� | +2 | +3 | +2 | +6��-2 | -2 |
| A�� | M��T�γɵĻ������۷е�� | |
| B�� | ����������Ӧ��ˮ������ԣ�L��Q | |
| C�� | �⻯��Ļ�ԭ�ԣ�H2 R��H2 T | |
| D�� | ���Ӱ뾶��T2-��L2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
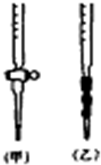 �Ķ�����ʵ�����ݣ�������ĿҪ��ش����⣮
�Ķ�����ʵ�����ݣ�������ĿҪ��ش����⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����NaOH��Һ��Ӧ��Al+6OH-�T[Al��OH��4]-+H2�� | |
| B�� | ��AlCl3��Һ�е��������ˮ��Al3++4NH3•H2O�T[Al��OH��4]-+4NH4+ | |
| C�� | Al2O3��NaOH��Һ��Ӧ��Al2O3+2OH-+3H2O�T2[Al��OH��4]- | |
| D�� | Ba��OH��2��Һ��������Һ��Ӧ��Al3+��ȫ������Ba2++3OH-+Al3++SO42-�TAl��OH��3��+BaSO4�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na+�Ĺ����ʾʽ�� | B�� | Na+�Ľṹʾ��ͼ�� | ||
| C�� | Na�ĵ����Ų�ʽ��1s22s22p63s1 | D�� | Na�ļ����Ų�ʽ��[Ne]3s1 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com