
��14�֣���ѧ�ϳ���ȼ�շ�ȷ���л������ɡ���ͼװ������ȼ�շ�ȷ���������ĺ������������ʽ�ij���װ�ã����ַ������ڵ�¯����ʱ�ô�������������Ʒ�����ݲ��������ȷ���л������ɡ�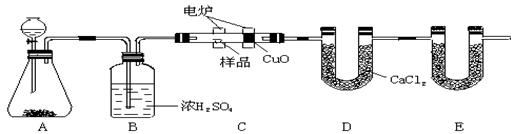
�ش��������⣺
��1��װ��A��װ��MnO2��д��Aװ���з�����Ӧ�Ļ�ѧ����ʽ�� ��
��2��Cװ��(ȼ�չ�)��CuO�������� ��
��3��д��Eװ������ʢ�����ʵ����� ������������ ��
��4������Bװ��ȥ�����ʵ�������ʲôӰ��? ��
��5����ȷ��ȡ1.20 g��Ʒ(�������ĺ���������)�������ȼ�պ�E����������1.76 g��D����������0.72 g������л����ʵ��ʽΪ ��
��6���Ӷ����ⶨȷ�Ƕ�ȥ���ǣ���װ��Ӧ������һ���Ľ�
��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| MnO2 |
| MnO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com