

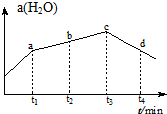
·ÖĪö £Ø1£©ŹģĻ¤ŅĒĘ÷µÄŠĪד£¬Ėµ³öĘäĆū³Ę£»
£Ø2£©1000mLČŻĮæĘæŌŚŹ¹ÓĆĒ°ŅŖ¼ģ²éŹĒ·ńĀ©Ė®£»
£Ø3£©ŅĄ¾ŻÕōĮóŹµŃéÓƵ½µÄŅĒĘ÷£ŗ¾Ę¾«µĘ”¢ŹÆĆŽĶų”¢ÕōĮóÉÕĘ攢ĪĀ¶Č¼Ę”¢ĄäÄż¹Ü”¢Å£½Ē¹Ü”¢×¶ŠĪĘ棬½ā“š£¬ĄäÄż¹ÜĖ®Į÷·½Ļņ£ŗĻĀæŚ½ųÉĻæŚ³ö£»
£Ø4£©ČŻĮæĘæÖ»ÄÜÅäÖĆ¹Ģ¶ØĢå»żµÄČÜŅŗ£»Ēćµ¹ČÜŅŗÓ¦ÓĆ²£Į§°ōŅżĮ÷£»
£Ø5£©øł¾ŻÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄČÜŅŗ²½Öč¶Ōø÷²Ł×÷½ųŠŠÅÅŠņ£»
£Ø6£©øł¾Ż×óÅĢµÄÖŹĮæ=ÓŅÅĢµÄÖŹĮæ+ÓĪĀėµÄÖŹĮæ½ā“š£»
ŅĄ¾Żm=CVM¼ĘĖćÓ¦³ĘČ”ĒāŃõ»ÆÄʵÄÖŹĮ棻
£Ø7£©·ÖĪö²»µ±²Ł×÷¶ŌČÜÖŹµÄĪļÖŹµÄĮæŗĶČÜŅŗĢå»żµÄÓ°Ļģ£¬ŅĄ¾ŻC=$\frac{n}{V}$½ųŠŠĪó²ī·ÖĪö£®
½ā“š ½ā£ŗ£Ø1£©×°ÖĆ¢ŁŹĒÕōĮóÉÕĘ棬װÖĆ¢ŚŹĒĄäÄż¹Ü£¬¹Ź“š°øĪŖ£ŗÕōĮóÉÕĘ棻ĄäÄż¹Ü£»
£Ø2£©1000mLČŻĮæĘæŌŚŹ¹ÓĆĒ°ŅŖ¼ģ²éŹĒ·ńĀ©Ė®£¬¹Ź“š°øĪŖ£ŗ¢Ü£»
£Ø3£©ÕōĮóŹµŃéÓƵ½µÄŅĒĘ÷£ŗ¾Ę¾«µĘ”¢ŹÆĆŽĶų”¢ÕōĮóÉÕĘ攢ĪĀ¶Č¼Ę”¢ĄäÄż¹Ü”¢Å£½Ē¹Ü”¢×¶ŠĪĘ棬ĖłŅŌȱɣµÄŅĒĘ÷ĪŖ¾Ę¾«µĘ£¬ĄäÄż¹ÜĖ®Į÷·½Ļņ£ŗĻĀæŚ½ųÉĻæŚ³ö£»
¹Ź“š°øĪŖ£ŗ¾Ę¾«µĘ£»g£»
£Ø4£©ÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄČÜŅŗŹĒ±ŲŠėÓĆ²£Į§°ōŅżĮ÷£¬·ĄÖ¹ŅŗĢåĶā½¦£¬ÅäÖʶą“óĢå»żµÄČÜŅŗŌņŃ”ŌńĻąÓ¦¹ęøńµÄČŻĮæĘ棻
¹Ź“š°øĪŖ£ŗĪ“ÓĆ²£Į§°ōŅżĮ÷£»Ī“²ÉÓĆ250mLČŻĮæĘ棻
£Ø5£©ÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄČÜŅŗ²½ÖčĪŖ£ŗ¼ĘĖć”¢³ĘĮ攢Čܽā”¢ĄäČ“”¢ŅĘŅŗ”¢Ļ“µÓ”¢¶ØČŻ”¢Ņ”ŌČµČ£¬ĖłŅŌÕżČ·µÄ²Ł×÷Ė³ŠņĪŖ£ŗ¢Ś¢Ł¢Ū¢ą¢Ż¢Ž¢ß¢Ü£¬
¹Ź“š°øĪŖ£ŗ¢Ś¢Ł¢Ū¢ą¢Ż¢Ž¢ß¢Ü£»
£Ø6£©ÓÉ×óÅĢµÄÖŹĮæ=ÓŅÅĢµÄÖŹĮæ+ÓĪĀėµÄÖŹĮææÉÖŖ£ŗķĄĀėÖŹĮæ=ÉÕ±ÖŹĮæ+ÓĪĀėµÄÖŹĮ棬ĖłŅŌÉÕ±ÖŹĮæ=ķĄĀėÖŹĮæ-ÓĪĀėÖŹĮ棬¼“ÉÕ±ÖŹĮæ=20g+10g-2.6g=27.4g£»ŹµŃéŹŅƻӊ240mLČŻĮæĘ棬Źµ¼ŹÉĻÅäÖʵďĒ250mL 1mol/LµÄĒāŃõ»ÆÄĘČÜŅŗ£¬ŠčŅŖĒāŃõ»ÆÄʵÄĪļÖŹµÄĮæĪŖ£ŗ1mol/L”Į0.25L=0.25mol£¬ŠčŅŖĒāŃõ»ÆÄʵÄÖŹĮæĪŖ£ŗ40g/mol”Į0.25mol=10.0g£¬
¹Ź“š°øĪŖ£ŗ27.4£»10.0£»
£Ø7£©¢ŁĆ»ÓŠĻ“µÓÉÕ±ŗĶ²£Į§°ō£¬µ¼ÖĀČÜÖŹµÄĪļÖŹµÄĮæĘ«Š”£¬ČÜŅŗÅضČĘ«µĶ£¬¹Ź²»Ń”£»
¢Ś×ŖŅĘČÜŅŗŹ±²»É÷ÓŠÉŁĮæČÜŅŗČ÷µ½ČŻĮæĘæĶāĆę£¬µ¼ÖĀČÜÖŹµÄĪļÖŹµÄĮæĘ«Š”£¬ČÜŅŗÅضČĘ«µĶ£¬¹Ź²»Ń”£»
¢ŪČŻĮæĘæ²»øÉŌļ£¬ŗ¬ÓŠÉŁĮæÕōĮóĖ®£¬¶ŌČÜÖŹµÄĪļÖŹµÄĮæŗĶČÜŅŗĢå»ż¶¼²»²śÉśÓ°Ļģ£¬ČÜŅŗÅØ¶Č²»±ä£¬¹Ź²»Ń”£»
¢Ü¶ØČŻŹ±ø©ŹÓæĢ¶ČĻߣ¬µ¼ÖĀČÜŅŗĢå»żĘ«Š”£¬ČÜŅŗÅضČĘ«øߣ¬¹ŹŃ”£»
¢Ż¶ØČŻŅ”ŌČŗó·¢ĻÖČÜŅŗĢå»żµĶÓŚæĢ¶ČĻߣ¬ŌŁ²¹¼ÓÉŁĮæÕōĮóĖ®ÖĮæĢ¶ČĻߣ¬µ¼ÖĀČÜŅŗĢå»żĘ«“ó£¬ČÜŅŗÅضČĘ«µĶ£¬¹Ź²»Ń”£»
¹ŹŃ”£ŗ¢Ü£®
µćĘĄ ±¾Ģāæ¼²éĮĖÕōĮóŹµŃ飬Ņ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗµÄÅäÖĘ£¬Ć÷Č·ÕōĮóŹµŃéµÄŌĄķŗĶŅĒĘ÷Ź¹ÓĆ×¢ŅāŹĀĻī¼°ÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗµÄŌĄķŹĒ½āĢā¹Ų¼ü£¬ĢāÄæÄŃ¶Č²»“ó£®


 Ó¦ÓĆĢāĢģĢģĮ·ĖÄ“Ø“óѧ³ö°ęÉēĻµĮŠ“š°ø
Ó¦ÓĆĢāĢģĢģĮ·ĖÄ“Ø“óѧ³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ”°µĶĢ¼Ń»·”±”¢ČēŗĪ½µµĶ“óĘųÖŠCO2µÄŗ¬Į攢ӊŠ§µŲæŖ·¢ĄūÓĆCO2£¬ŅżĘšĮĖČ«ŹĄ½ēµÄĘÕ±éÖŲŹÓ£®
”°µĶĢ¼Ń»·”±”¢ČēŗĪ½µµĶ“óĘųÖŠCO2µÄŗ¬Į攢ӊŠ§µŲæŖ·¢ĄūÓĆCO2£¬ŅżĘšĮĖČ«ŹĄ½ēµÄĘÕ±éÖŲŹÓ£®| ŹµŃé×é | ĪĀ¶Č/”ę | ĘšŹ¼Įæ£Ømol£© | Ę½ŗāĮæ£Ømol£© | “ļµ½Ę½ŗāĖł ŠčŅŖŹ±¼ä/min | ||
| CO£Øg£© | H2O£Øg£© | CO2£Øg£© | H2£Øg£© | |||
| I | 800 | 2 | 2 | x | 1 | 5 |
| II | 900 | 1 | 2 | 0.5 | 0.5 | tm |
| III | 900 | 2 | 4 | y | y | tn |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĪĀ¶ČÉżøߣ¬Na2CO3ČÜŅŗpH¼õŠ” | |
| B£® | ĒāŃõ»ÆÄĘČÜŅŗ¾ĆÖĆÓŚæÕĘųÖŠ£¬ČÜŅŗpH±ä“ó | |
| C£® | ŠĀÖĘĀČĖ®¾¹āÕÕŅ»¶ĪŹ±¼äŗó£¬ČÜŅŗpH¼õŠ” | |
| D£® | ĪĀ¶ČÉżøߣ¬“æĖ®pHŌö“ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 2Na+2NH3ØT2NaNH2+H2”ü | B£® | 2NH3+3CuOØT3Cu+N2+3H2O | ||
| C£® | NH3+H2O?NH3•H2O | D£® | HCl+NH3ØTNH4Cl |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
 ”°“ļ·Ę”±ČšŹæĀŽŹĻÖĘŅ©¹«Ė¾Éś²śµÄŅ»ÖÖÖĪĮĘĒŻĮ÷øŠŗĶ¼×ŠĶH1N1Į÷øŠ£ØÖķĮ÷øŠ£©µÄĢŲŠ§Ņ©£®“ļ·ĘµÄÖ÷ŅŖÓŠŠ§³É·ÖƧ²ŻĖįŹĒ“ÓÖŠ¹ś°ŁŠÕ³£¼ūµÄµ÷Ī¶ĮĻ°Ė½ĒÜīĻćÖŠĢįČ”³öĄ“µÄ£®Ć§²ŻĖįµÄ½į¹¹Ź½ČēĶ¼£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©
”°“ļ·Ę”±ČšŹæĀŽŹĻÖĘŅ©¹«Ė¾Éś²śµÄŅ»ÖÖÖĪĮĘĒŻĮ÷øŠŗĶ¼×ŠĶH1N1Į÷øŠ£ØÖķĮ÷øŠ£©µÄĢŲŠ§Ņ©£®“ļ·ĘµÄÖ÷ŅŖÓŠŠ§³É·ÖƧ²ŻĖįŹĒ“ÓÖŠ¹ś°ŁŠÕ³£¼ūµÄµ÷Ī¶ĮĻ°Ė½ĒÜīĻćÖŠĢįČ”³öĄ“µÄ£®Ć§²ŻĖįµÄ½į¹¹Ź½ČēĶ¼£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©| A£® | ĖüÄÜŹ¹äåĖ®ĶŹÉ« | |
| B£® | ÓöFeCl3ČÜŅŗ³Ź×ĻÉ« | |
| C£® | Ėü²»ÄÜ·¢ÉśĻūČ„·“Ó¦ | |
| D£® | 1molƧ²ŻĖįøś×ćĮæµÄÄĘ·“Ó¦æÉŅŌµĆµ½4molH2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Ę½ŗāĻņÕż·“Ó¦·½ĻņŅʶÆĮĖ | B£® | ĪļÖŹAµÄ×Ŗ»ÆĀŹ¼õÉŁĮĖ | ||
| C£® | ĪļÖŹCµÄÖŹĮæ·ÖŹżŌö¼ÓĮĖ | D£® | a+b£¼c |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | H2O | B£® | H2O2 | C£® | 11H”¢21H | D£® | 1940CaŗĶ2040Ca |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
 ÄÉĆײÄĮĻTiO2ŌŚĶæĮĻ”¢¹ā“߻Ɣ¢»ÆױʷµČĮģÓņӊ׿«Ęä¹ć·ŗµÄÓ¦ÓĆ£®ÖʱøÄÉĆ×TiO2µÄ·½·ØÖ®Ņ»ŹĒTiCl4ŌŚ¼ÓČČĢõ¼žĻĀĖ®½āÉś³ÉTiO2•xH2O£¬¾¹ż¹żĀĖ”¢Ė®Ļ“³żČ„ĘäÖŠµÄCl-£¬ŌŁ¾ŗęøÉ”¢±ŗÉÕ³żČ„Ė®·Ö£¬×īŗóµĆµ½·ŪĢåTiO2£®
ÄÉĆײÄĮĻTiO2ŌŚĶæĮĻ”¢¹ā“߻Ɣ¢»ÆױʷµČĮģÓņӊ׿«Ęä¹ć·ŗµÄÓ¦ÓĆ£®ÖʱøÄÉĆ×TiO2µÄ·½·ØÖ®Ņ»ŹĒTiCl4ŌŚ¼ÓČČĢõ¼žĻĀĖ®½āÉś³ÉTiO2•xH2O£¬¾¹ż¹żĀĖ”¢Ė®Ļ“³żČ„ĘäÖŠµÄCl-£¬ŌŁ¾ŗęøÉ”¢±ŗÉÕ³żČ„Ė®·Ö£¬×īŗóµĆµ½·ŪĢåTiO2£®²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com