
���۸�[Cr������]�о綾����ˮ�е�Cr2O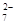 �����������巨��ȥ�����ѹ�����FeSO4•7H2O���뺬Cr2O
�����������巨��ȥ�����ѹ�����FeSO4•7H2O���뺬Cr2O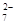 �ķ�ˮ�У�����pH��4��Fe2����ԭCr������ΪCr3����
�ķ�ˮ�У�����pH��4��Fe2����ԭCr������ΪCr3����
��1��д��Fe2����ԭCr2O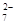 �����ӷ���ʽ������������ת�Ƶķ������Ŀ��
�����ӷ���ʽ������������ת�Ƶķ������Ŀ��
_________________________________________________________��
��2�����ڣ�1���õ���Һ��pHֵΪ8��10���������൱��Fe����[Fe����xCr2��x]O4�����Բ��������壩�ij������ɣ�1����ȷ��x��_______��
��3��Cr3���ڹ�����NaOH��Һ�л�ת��ΪCrO��д����һת�������ӷ���ʽ_______________________________���ɴ˿�֪Cr��OH��3��_________��ѡ��ᡱ��������������ԡ�
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| NaOH |
| Cl2 |
| �ữ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���Ϻ��г�����ѧ2012�������ѧ�����п��Ի�ѧ���� ���ͣ�022
���۸�[Cr(��)]�о綾����ˮ�е�Cr2O![]() �����������巨��ȥ�����ѹ�����FeSO4��7H2O���뺬Cr2O
�����������巨��ȥ�����ѹ�����FeSO4��7H2O���뺬Cr2O![]() �ķ�ˮ�У�����pH��4��Fe2+��ԭCr(��)ΪCr3+��
�ķ�ˮ�У�����pH��4��Fe2+��ԭCr(��)ΪCr3+��
(1)д��Fe2+��ԭCr2O![]() �����ӷ���ʽ������������ת�Ƶķ������Ŀ��
�����ӷ���ʽ������������ת�Ƶķ������Ŀ��
________��
(2)����(1)�õ���Һ��pHֵΪ8��10���������൱��Fe(��)[Fe(��)xCr2��x]O4(���Բ���������)�ij�������(1)��ȷ��x��________��
(3)Cr3+�ڹ�����NaOH��Һ�л�ת��ΪCrO![]() ��д����һת�������ӷ���ʽ________���ɴ˿�֪Cr(OH)3��________(ѡ��ᡱ�����������)�ԣ�
��д����һת�������ӷ���ʽ________���ɴ˿�֪Cr(OH)3��________(ѡ��ᡱ�����������)�ԣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���۸�[Cr������]�о綾����ˮ�е�Cr2O�����������巨��ȥ�����ѹ�����FeSO4•7H2O���뺬Cr2O
�ķ�ˮ�У�����pH��4��Fe2����ԭCr������ΪCr3����
��1��д��Fe2����ԭCr2O�����ӷ���ʽ������������ת�Ƶķ������Ŀ��
_________________________________________________________��
��2�����ڣ�1���õ���Һ��pHֵΪ8��10���������൱��Fe����[Fe����xCr2��x]O4�����Բ��������壩�ij������ɣ�1����ȷ��x��_______��
��3��Cr3���ڹ�����NaOH��Һ�л�ת��ΪCrO��д����һת�������ӷ���ʽ_______________________________���ɴ˿�֪Cr��OH��3��_________��ѡ��ᡱ��������������ԡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���Ϻ��г�����ѧ������ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
���۸�[Cr������]�о綾����ˮ�е�Cr2O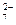 �����������巨��ȥ�����ѹ�����FeSO4?7H2O���뺬Cr2O
�����������巨��ȥ�����ѹ�����FeSO4?7H2O���뺬Cr2O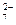 �ķ�ˮ�У�����pH��4��Fe2����ԭCr������ΪCr3����
�ķ�ˮ�У�����pH��4��Fe2����ԭCr������ΪCr3����
��1��д��Fe2����ԭCr2O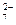 �����ӷ���ʽ������������ת�Ƶķ������Ŀ��
�����ӷ���ʽ������������ת�Ƶķ������Ŀ��
_________________________________________________________��
��2�����ڣ�1���õ���Һ��pHֵΪ8��10���������൱��Fe����[Fe����xCr2��x]O4�����Բ��������壩�ij������ɣ�1����ȷ��x��_______��
��3��Cr3���ڹ�����NaOH��Һ�л�ת��ΪCrO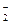 ��д����һת�������ӷ���ʽ_______________________________���ɴ˿�֪Cr��
��д����һת�������ӷ���ʽ_______________________________���ɴ˿�֪Cr�� OH��3��_________��ѡ��ᡱ��������������ԡ�
OH��3��_________��ѡ��ᡱ��������������ԡ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com