
°ŃŌŚæÕĘųÖŠ¾ĆÖƵÄĀĮʬ5.0gĶ¶ČėŹ¢ÓŠ500mL 0.5mol”¤L-1ĮņĖįČÜŅŗµÄÉÕ±ÖŠ£¬øĆĀĮʬÓėĮņĖį·“Ó¦²śÉśĒāĘųµÄĖŁĀŹÓė·“Ó¦Ź±¼äæÉÓĆČēÓŅµÄ×ų±źĒśĻߥ“±ķŹ¾£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
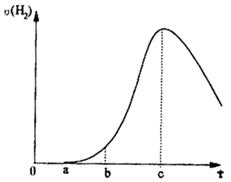
1.ĒśĻßÓÉ0”śa¶Ī²»²śÉśĒāĘųµÄŌŅņ£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£¬
ÓŠ¹ŲµÄĄė×Ó·½³ĢŹ½ĪŖ£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£»
2.ĒśĻßÓÉa”śb¶Ī²śÉśĒāĘųµÄĖŁĀŹ½ĻĀżµÄŌŅņ£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£»
3.ĒśĻßÓÉb”śc¶Ī£¬²śÉśĒāĘųµÄĖŁĀŹŌö¼Ó½ĻæģµÄÖ÷ŅŖŌŅņ£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£»
4.ĒśĻßÓÉcŅŌŗ󣬲śÉśĒāĘųµÄĖŁĀŹÖš½„ĻĀ½µµÄÖ÷ŅŖŌŅņ£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß”£
1.ĀĮʬ±ķĆęÓŠŃõ»ÆĀĮ£¬ĮņĖįŹ×ĻČÓė±ķĆęµÄŃõ»ÆĀĮ·“Ó¦£»
Al2O3 + 6H+ = 2Al3+ + 3H2O£»
2.æŖŹ¼Ź±£¬ĮņĖįÓėĀĮʬֻӊ²æ·Ö×÷ÓĆ£¬ČÜŅŗĪĀ¶ČµĶ£»
3.·“Ó¦·Å³öµÄČČĮæŹ¹ČÜŅŗµÄĪĀ¶ČÉżø߶ų¼Óæģ·“Ó¦ĖŁĀŹ£»
4.Ėę×Å·“Ó¦µÄ½ųŠŠ£¬ĮņĖįČÜŅŗÅضČĻĀ½µ”£
”¾½āĪö”æ
1.ĀĮŹōÓŚ»īĘĆµÄ½šŹō£¬Ęä±ķĆę±»Ńõ»ÆÉś³ÉĮĖŅ»²ćÖĀĆܵÄŃõ»ÆĀĮ£¬ĖłŅŌĶ¶Čėµ½ĮņĖįÖŠ£¬ĮņĖįŹ×ĻČÓė±ķĆęµÄŃõ»ÆĀĮ·“Ó¦£¬Č»ŗóŌŁŗĶµ„ÖŹĀĮ·“Ӧɜ³ÉĒāĘų£¬·“Ó¦Ź½ĪŖAl2O3 + 6H+ = 2Al3+ + 3H2O”£
2.ÓÉÓŚæŖŹ¼·“Ó¦Ź±ČÜŅŗµÄĪĀ¶Č±Č½ĻµĶ£¬·“Ó¦ĖŁĀŹ¾ĶĀż”£
3.ŅņĪŖĀĮŗĶĮņĖį·“Ó¦ŹōÓŚ·ÅČČ·“Ó¦£¬Ėę×Å·“Ó¦µÄ½ųŠŠ£¬ČÜŅŗµÄĪĀ¶ČÖš½„Éżøߣ¬ĖłŅŌ·“Ó¦ĖŁĀŹÖš½„¼Óæģ”£
4.µ±·“Ó¦½ųŠŠµ½Ņ»¶Ø³Ģ¶ČŹ±ĮņĖįµÄÅØ¶Č½µµĶ£¬¼““ĖŹ±ÅØ¶ČµÄÓ°Ļģ³¬¹żĮĖĪĀ¶Č¶Ō·“Ó¦ĖŁĀŹµÄÓ°Ļģ£¬ĖłŅŌ·“Ó¦ĖŁĀŹŅŖÖš½„½µµĶ”£


 æŖŠÄĮ·Ļ°æĪæĪĮ·Óėµ„ŌŖ¼ģ²āĻµĮŠ“š°ø
æŖŠÄĮ·Ļ°æĪæĪĮ·Óėµ„ŌŖ¼ģ²āĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 °ŃŌŚæÕĘųÖŠ¾ĆÖƵÄĀĮʬ5.0gĶ¶ČėŹ¢ÓŠ500mL0£¬5mol?L-1ŃĪĖįČÜŅŗµÄÉÕ±ÖŠøĆĀĮʬÓėŃĪĖį·“Ó¦²śÉśĒāĘųµÄĖŁĀŹÓė·“Ó¦Ź±¼äæÉÓĆČēĶ¼ĖłŹ¾µÄ×ų±źĒśĻߥ“±ķŹ¾£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
°ŃŌŚæÕĘųÖŠ¾ĆÖƵÄĀĮʬ5.0gĶ¶ČėŹ¢ÓŠ500mL0£¬5mol?L-1ŃĪĖįČÜŅŗµÄÉÕ±ÖŠøĆĀĮʬÓėŃĪĖį·“Ó¦²śÉśĒāĘųµÄĖŁĀŹÓė·“Ó¦Ź±¼äæÉÓĆČēĶ¼ĖłŹ¾µÄ×ų±źĒśĻߥ“±ķŹ¾£¬»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 £Ø2011?Ó„Ģ¶¶žÄ££©°ŃŌŚæÕĘųÖŠ¾ĆÖƵÄĀĮʬ5.0gĶ¶ČėŹ¢ÓŠ 500mL 0.5mol?L-1ĮņĖįČÜŅŗµÄÉÕ±ÖŠ£¬øĆĀĮʬÓėĮņĖį·“Ó¦²śÉśĒāĘųµÄĖŁĀŹvÓė·“Ó¦Ź±¼ätæÉÓĆČēĶ¼µÄ×ų±źĒśĻߥ“±ķŹ¾£¬ĻĀĮŠĶĘĀŪ“ķĪóµÄŹĒ£Ø””””£©
£Ø2011?Ó„Ģ¶¶žÄ££©°ŃŌŚæÕĘųÖŠ¾ĆÖƵÄĀĮʬ5.0gĶ¶ČėŹ¢ÓŠ 500mL 0.5mol?L-1ĮņĖįČÜŅŗµÄÉÕ±ÖŠ£¬øĆĀĮʬÓėĮņĖį·“Ó¦²śÉśĒāĘųµÄĖŁĀŹvÓė·“Ó¦Ź±¼ätæÉÓĆČēĶ¼µÄ×ų±źĒśĻߥ“±ķŹ¾£¬ĻĀĮŠĶĘĀŪ“ķĪóµÄŹĒ£Ø””””£©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
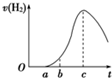 °ŃŌŚæÕĘųÖŠ¾ĆÖƵÄĀĮʬ5.0gĶ¶ČėŹ¢ÓŠ500mL 0.5mol?L-1ĮņĖįČÜŅŗµÄÉÕ±ÖŠ£¬øĆĀĮʬÓėĮņĖį·“Ó¦²śÉśĒāĘųµÄĖŁĀŹÓė·“Ó¦Ź±¼äµÄ¹ŲĻµæÉÓĆČēĶ¼ĒśĻߥ“±ķŹ¾£®
°ŃŌŚæÕĘųÖŠ¾ĆÖƵÄĀĮʬ5.0gĶ¶ČėŹ¢ÓŠ500mL 0.5mol?L-1ĮņĖįČÜŅŗµÄÉÕ±ÖŠ£¬øĆĀĮʬÓėĮņĖį·“Ó¦²śÉśĒāĘųµÄĖŁĀŹÓė·“Ó¦Ź±¼äµÄ¹ŲĻµæÉÓĆČēĶ¼ĒśĻߥ“±ķŹ¾£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
°ŃŌŚæÕĘųÖŠ¾ĆÖƵÄĀĮʬ5.0 gĶ¶ČėŹ¢ÓŠ 500mL0.5 mol”¤L-1ĮņĖįČÜŅŗµÄÉÕ±ÖŠ£¬øĆĀĮʬÓėĮņĖį·“Ó¦²śÉśĒāĘųµÄĖŁĀŹÓė·“Ó¦Ź±¼äæÉÓĆČēÓŅµÄ×ų±źĒśĻߥ“±ķŹ¾£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ĒśĻßÓÉ0”śa¶Ī²»²śÉśĒāĘųµÄŌŅņ___________,
ÓŠ¹Ų·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ____________ £»
£Ø2£©ĒśĻßÓÉa”śb¶Ī²śÉśĒāĘųµÄĖŁĀŹ½ĻĀżµÄŌŅņ___________
ÓŠ¹ŲµÄ»Æѧ·½³ĢŹ½__________________________£»
£Ø3£©ĒśĻßÓÉb”śc¶Ī£¬²śÉśĒāĘųµÄĖŁĀŹŌö¼Ó½ĻæģµÄÖ÷ŅŖŌŅņ_________________________£»
£Ø4£©ĒśĻßÓÉcŅŌŗ󣬲śÉśĒāĘųµÄĖŁĀŹÖš½„ĻĀ½µµÄÖ÷ŅŖŌŅņ_________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģɽ¶«Ź”øßŅ»ĻĀŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø8·Ö£©°ŃŌŚæÕĘųÖŠ¾ĆÖƵÄĀĮʬ5.0 gĶ¶ČėŹ¢ÓŠ500 mL 0.5 mol”¤L£1ĮņĖįČÜŅŗµÄÉÕ±ÖŠ£¬øĆĀĮʬÓėĮņĖį·“Ó¦²śÉśĒāĘųµÄĖŁĀŹÓė·“Ó¦Ź±¼äµÄ¹ŲĻµæÉÓĆČēĶ¼ĒśĻߥ“±ķŹ¾£¬»Ų“šĻĀĮŠĪŹĢā£ŗ

(1)ĒśĻßÓÉO ”śa¶Ī²»²śÉśĒāĘųµÄŌŅņŹĒ____________________________________________________________£»
ÓŠ¹Ų·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
__________________________________________________£»
(2)ĒśĻßa”śc¶Ī£¬²śÉśĒāĘųµÄĖŁĀŹŌö¼Ó½ĻæģµÄÖ÷ŅŖŌŅņŹĒ
________________________________________________________________________
________________________________________________________________________£»
(3)ĒśĻßÓÉcŅŌŗ󣬲śÉśĒāĘųµÄĖŁĀŹÖš½„ĻĀ½µµÄÖ÷ŅŖŌŅņŹĒ_________________________£»
(4)øĆ·“Ó¦ČōŹ¹ÓĆ“ß»Æ¼Į£¬æÉŹ¹H2²śĮæŌö¶ąĀš£æ______(Ģī”°»į”±»ņ”°²»»į”±)”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com