

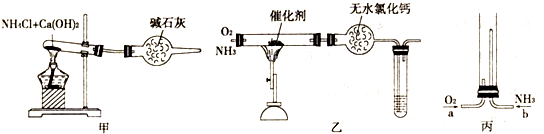
��1����װ��A��ȡ����������İ��������Թ���̼���εĻ�ѧʽ��__________________����ʯ�ҵ�������________________________________________________________________��
��2���������İ��������������ͨ��װ��B������Ϊ��ʯ�ޣ��У��þƾ���Ƽ��ȣ�
�ٰ��������Ļ�ѧ����ʽ��____________________________________���Թ��������Ϊ����ɫ���÷�Ӧ�Ļ�ѧ����ʽ��______________________________________________��
��ֹͣ��Ӧ�������ر�B������������һ��ʱ����Թܽ����ˮ��������һ�ֶ�����NO2��Է��������Ļ�����Թ���������ɫ��dz�����ϻ�ѧ����ʽ˵��ԭ��__________
_____________________________________________________________________��
��3����������������A�����İ����ֱ��a��b���ܽ�����ͨ�뵽װ��C�У���b���϶˵�ȼ����δ������ɫ���壺
��������ͨ����Ⱥ�˳����_______________����������__________________________��
�ڰ���ȼ�յĻ�ѧ����ʽ��________________________________________________��
˼·��������1���ư�����̼���κ��������ǣ�NH4��2CO3��NH4HCO3����NH4��2CO3��NH4HCO3���ȷֽ�Ļ�ѧ����ʽΪ��![]() ��
��![]()
��Ȼ����ʯ�ҵ�����������CO2��H2O��
��2���ٰ��������ڲ�������������ȼ�գ�������NO��H2O�����ǰ�������������ĵ�һ��������������Ҳ˵������һ�㡣
![]()
�Թ��ڱ����ɫ���϶���NO�������ɵ�NO2��2NO+O2![]() 2NO2
2NO2
�ڿɴ��Ʋ⣬������NO2��Է��������Ļ�������N2O4������������ɫ�ģ����ҵ��������������ɡ��Թ�����ɫ��dz��˵��NO2������ȫת��ΪN2O4������NO2����N2O4�ķ�Ӧ����ʽӦ�ǣ�
2NO2![]() N2O4��
N2O4��
��3����Ӧ��ͨ����������ͨ�백�� �������Ե�ȼ�����Ұ�������Ⱦ����
�ڰ���û�д����������ȼ�գ���δ���к���ɫ�������ɣ��϶���������NO����Ӧ���ǵ�����4NH3+3O2![]() 2N2+6H2O
2N2+6H2O
�𰸣���1����NH4��2CO3��NH4HCO3 ![]() ��
��![]()
��2���� ![]()
2NO+O2![]() 2NO2
2NO2
�ڿɴ��Ʋ⣬������NO2��Է��������Ļ�������N2O4������������ɫ�ģ����ҵ��������������ɡ��Թ�����ɫ��dz��˵��NO2������ȫת��ΪN2O4������NO2����N2O4�ķ�Ӧ����ʽӦ�ǣ�
2NO2![]() N2O4��
N2O4��
��3����Ӧ��ͨ����������ͨ�백�� �������Ե�ȼ�����Ұ�������Ⱦ������
��4NH3+3O2![]() 2N2+6H2O
2N2+6H2O



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| �� |
| ||
| �� |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| �� |
| ||
| �� |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����������ѧ�߶���ѧ�����п������ƻ�ѧ�Ծ����������� ���ͣ�ʵ����
������ʾ�����������ڴ����а���ȼ�ա�������ijУ��ѧС��ѧ���������װ�ã�ͼ�����еȼг�װ������ȥ�����а����������ڲ�ͬ�����·�Ӧ��ʵ�顣
��1�������ӵĿռ�ṹ�� ���������������������ͨ��װ��B������Ϊ��ʯ�ޣ��У��þƾ���Ƽ��Ƚ��а��Ĵ��������Թ��ڳ��ֵ������� ��
��2��ֹͣ��Ӧ�������ر�B������������һ��ʱ����Թܽ����ˮ�У��Թ���������ɫ��dz�����ϻ�ѧ����ʽ˵��ԭ�� ��
��3����������������A�����İ����ֱ��a��b���ܽ�����ͨ�뵽װ��C�У�����b���϶˵�ȼ������������ͨ����Ⱥ�˳��Ӧ���� ��
��4����֪�����ڴ�����ȼ�գ���Ԫ��ȫ��ת����N2����д���÷�Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ�����ص�һ��ѧ��һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
������ʾ�����������ڴ����а���ȼ�գ�����������Ⱦ�IJ��������ijУ��ѧС��ѧ�������ͼװ��(ͼ�����еȼг�װ������ȥ)���а����������ڲ�ͬ�����·�Ӧ��ʵ�顣
(1)��װ��A��ȡ����������İ��������Թ���̼���εĻ�ѧʽ��________����ʯ�ҵ�������__________________��
(2)�������İ��������������ͨ��װ��B(����Ϊ��ʯ��)�У��þƾ���Ƽ��ȣ����������Ļ�ѧ����ʽ��____________________________________���Թ��������Ϊ����ɫ���÷�Ӧ�Ļ�ѧ����ʽ��_________________________��
(3)��������������A�����İ����ֱ��a��b���ܽ�����ͨ�뵽װ��C�У�����b���϶˵�ȼ��������������ȼ�յĻ�ѧ����ʽ��___________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com