
ij������FeCl3��Һ��ʴ����ͭ�ľ�Ե������ӡˢ��·��������Ӧ�Ļ�ѧ����ʽΪ��2FeCl3��Cu��2FeCl2��CuCl2��ʵ��С���ͬѧ������ӡˢ��·��ķ�Һ�ɷֽ���������̽������ȡ������Һ���μ�KSCN��Һ�Ժ�ɫ����ȡ10 mL��Һ������������AgNO3��Һ����������8.61 g������ȡ10mL��Һ������һ��������ͭƬ����ַ�Ӧ���ͭƬ������������0.256 g������Ӧ�����Һ�еμ�KSCN��Һ����ɫ������ʵ��ó����ۣ�
��1����Һ�к��еĽ����������� ��
��2�������й����ݼ���ó�ʹ�õ�FeCl3��Һ�����ʵ����ʵ���Ũ��Ϊ_____________�����踯ʴ��·�����Һ������䣩��10 mL��Һ��ͭ���ӵ����ʵ���Ϊ ��
 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ�����з�خ������ͳ����һ�����ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
ij������Һ�к��б��ӡ����ᣬʵ��С��Ը÷�Һ����̽����������·�����
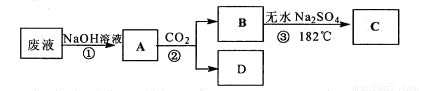
��֪�۵㣺����16.6�桢����43�档�е㣺����118�桢����182�档
��1��д���ڵķ�Ӧ��ѧ����ʽ ��
��2�������B�IJ��������� ��
��3���ֶ�����C�����ʽ���ʵ��̽�����������ʵ��С�鰴Ҫ�����ʵ����̼�¼���ڴ������д��ʵ�������Ԥ�������������͡�
��ѡ�Լ�������ˮ��ϡHNO3��2moL��L��1NaOH��0.1 mol •L��1KSCN������KMnO4�� Һ��FeCl3��Һ��������ˮ����ɫʯ����Һ��
|
ʵ����� |
Ԥ������ |
������� |
|
����1��ȡ����C����a�Թܣ�������������ˮ���� |
|
|
|
����2��ȡ����C��ϡ��Һ��װb��c��֧�Թܣ���b�Թ�
|
������ɫ����
|
|
|
����3����c�Թ�
|
|
C�������Լ�������ɫ��Ӧ�� |
��4����ȡһ������C��������ˮ�ܽ��ȫ��ת����1000mL����ƿ�ж��ݡ�ȡ����Һ 25.00mL������Ũ��Ϊ0.0500 moL��L��1����ˮ��Һ30.00mL�����á�����Ӧ��ȫ���������KI������0.1100 moL•L��1Na2S2O3����Һ�ζ����ɵ�I2����ȥNa2S2O3����Һ11.80mL����������C���ʵ����ļ������ʽΪ�� �� �����ַ�Ӧ���ӷ���ʽ��I2+2S2O32��=2I��+S4O62����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com