
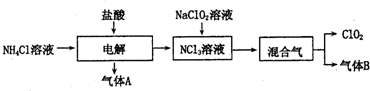
���� NH4Cl��Һ�м���������е�⣬�õ�NCl3�����Ԫ���غ��֪�����ɵ�����AΪH2����ⷢ����Ӧ��NH4Cl+2HCl$\frac{\underline{\;���\;}}{\;}$NCl3+3H2����NCl3��Һ�м���NaClO2��Һ���õ�ClO2������B������B��ʹʪ��ĺ�ɫʯ����ֽ��������BΪNH3��
��1��������������֪����ⷴӦ��NH4Cl��HCl�������NCl3��H2���ݴ���д��Ӧ����ʽ��ʵ�������Ȼ�������������ڼ����������Ʊ���������Ӧ�����Ȼ��ơ�������ˮ��
����Ŀ��Ϣ��֪��NCl3���ױ�ը��������ϡ���������ϡ�ͣ�����ˮ���ֽ⣬�����Ӧע�⣺���ƺ�����NCl3��Ũ�ȣ����ƺ÷�Ӧ�¶ȣ�
��2��NCl3��NaClO2�����ʵ���֮��Ϊ1��6��ϣ�����Һ��ǡ�÷�Ӧ����ClO2��NH3������Ԫ���غ��֪����ˮ�μӷ�Ӧ��1molNCl3�õ�1molNH3����ClO2-��ClO2��Clԭ����Oԭ��֮�Ⱦ�Ϊ1��2����ϵ���غ��֪����Ӧ������NaCl��NaOH��
��3���ٵⷴӦ��ϣ��������һ��Na2S2O3��Һ����Һ��ɫ��ȥ��
��ClO2��I-��Ӧ�õ�Cl-��I2���ɵ���ת���غ㼰��֪��Ӧ���ɵù�ϵʽ��2ClO2��5I2��10S2O32-���ݴ˼���V1 mL������ClO2�����ʵ�������������100mL��Һ��ClO2�����ʵ������ٽ��m=nM����ԭ10mL��Һ��ClO2��Ũ�ȣ�
��� �⣺��1��NH4Cl��Һ�м���������е�⣬�õ�NCl3�����Ԫ���غ��֪�����ɵ�����AΪH2����ⷢ����Ӧ��NH4Cl+2HCl$\frac{\underline{\;���\;}}{\;}$NCl3+3H2����ʵ�������Ȼ�������������ڼ����������Ʊ���������Ӧ�����Ȼ��ơ�������ˮ����Ӧ����ʽΪ��2NH4Cl+Ca��OH��2 $\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
����Ŀ��Ϣ��֪��NCl3���ױ�ը��������ϡ���������ϡ�ͣ�����ˮ���ֽ⣬�����Ӧע�⣺���ƺ�����NCl3��Ũ�ȣ����ƺ÷�Ӧ�¶ȣ�
�ʴ�Ϊ��NH4Cl+2HCl$\frac{\underline{\;���\;}}{\;}$NCl3+3H2����2NH4Cl+Ca��OH��2 $\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O�����ƺ÷�Ӧ�¶ȣ�
��2��NCl3��NaClO2�����ʵ���֮��Ϊ1��6��ϣ�����Һ��ǡ�÷�Ӧ����ClO2��NH3������Ԫ���غ��֪����ˮ�μӷ�Ӧ��1molNCl3�õ�1molNH3����ClO2-��ClO2��Clԭ����Oԭ��֮�Ⱦ�Ϊ1��2����ϵ���غ��֪����Ӧ������NaCl��NaOH����Ӧ���ӷ���ʽΪ��NCl3+6ClO2-+3H2O=6ClO2��+NH3��+3Cl-+3OH-��
�ʴ�Ϊ��NCl3+6ClO2-+3H2O=6ClO2��+NH3��+3Cl-+3OH-��
��3���ٵⷴӦ��ϣ��������һ��Na2S2O3��Һ����Һ��ɫ��Ϊ��ɫ����30s�ڲ���ɫ��˵����Ӧ�����յ㣬
�ʴ�Ϊ�����һ�ε������Һ����ɫ�����ɫ����30s�ڲ���ɫ��
��ClO2��I-��Ӧ�õ�Cl-��I2���ɵ���ת���غ㼰��֪��Ӧ���ɵù�ϵʽ��2ClO2��5I2��10S2O32-����V1 mL������ClO2�����ʵ���Ϊc mol/L��V2��10-3L��$\frac{1}{5}$����100mL��Һ��ClO2�����ʵ���Ϊc mol/L��V2��10-3L��$\frac{1}{5}$��$\frac{100mL}{{V}_{1}mL}$����ԭ10mL��Һ��ClO2������Ϊc mol/L��V2��10-3L��$\frac{1}{5}$��$\frac{100mL}{{V}_{1}mL}$��67.5g/mol=cV2��10-3��$\frac{1}{5}$��$\frac{100}{{V}_{1}}$��67.5g����ԭ��Һ��ClO2��Ũ��Ϊ��cV2��10-3��$\frac{1}{5}$��$\frac{100}{{V}_{1}}$��67.5g����0.01L=$\frac{135C{V}_{2}}{{V}_{1}}$g/L��
�ʴ�Ϊ��$\frac{135c{v}_{2}}{{V}_{1}}$��
���� ���⿼��ʵ�鷽������ƣ��漰�Է�Ӧԭ���Ŀ��顢�Է�Ӧ�����Ŀ��ơ�������ԭ��Ӧ�ζ�Ӧ�õȣ�ע�������Ŀ��Ϣ��֪��Ӧ�������ϺõĿ���ѧ���������⡢����������������Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
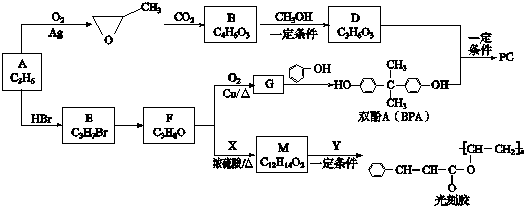
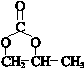 ��
��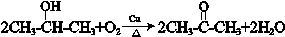 ��
��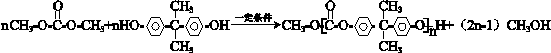 ��
��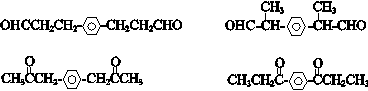 ������һ�֣���
������һ�֣��� �ṹ
�ṹ +
+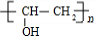 $\stackrel{һ������}{��}$
$\stackrel{һ������}{��}$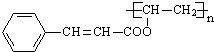 +nCH3CHOHCH3��
+nCH3CHOHCH3���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
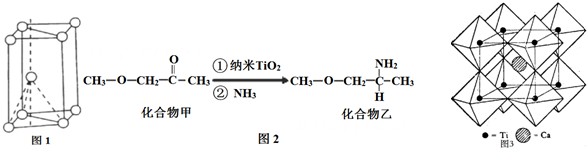
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
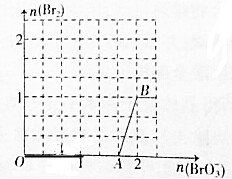
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.1mol/L NaHCO3��Һ��0.1mol/L NaOH��Һ�������ϣ�������Һ�У�C��Na+����c��CO32-����c��HCO3- ����c��OH-�� | |
| B�� | 20ml 0.1mol/L CH3COONa��Һ��10ml0.1mol/L HCl��Һ��Ϻ���Һ�����ԣ��������У�C��CH3COO-����c��Cl-����c��CH3COOH����c��H+�� | |
| C�� | �����£�pH=2��������pH=12�İ�ˮ�������ϣ�������Һ�У�c��Cl-��+c��H+����c��NH4+ ��+c��OH-�� | |
| D�� | 0.1mol/L CH3COOH��Һ��0.1mol/L NaOH��Һ�������ϣ�������Һ�У�c��OH-����c��H+��+c��CH3COOH�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | C��D���γ�D2C2�����ӻ����� | |
| B�� | ��A��Dԭ�ӹ��ɵķ��ӵĽṹ���������� | |
| C�� | E��Cֻ���γ�E2C һ�ֻ����� | |
| D�� | ��A��B��C��D����Ԫ���γɵĻ�������������ӻ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CO2�ĵ���ʽ 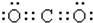 | |
| B�� | Cl-�Ľṹʾ��ͼ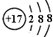 | |
| C�� | NaOH�ĵ���ʽ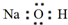 | |
| D�� | ԭ�Ӻ�����20�����ӵ���ԭ��${\;}_{17}^{37}$Cl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaCl | B�� | KCl | C�� | CaCl2 | D�� | AlCl3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com