
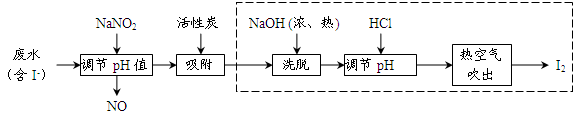


 ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ȥ�Ҵ���ˮ����������ʯ�ң������ռ������ |
| B��������۵�ˮ������������������ˮ��Һ��ֱ�Ӽ�������Cu(OH)2��Һ��Ȼ����ȣ��۲��Ƿ��к�ɫ�������� |
| C����ȥ���������е�����ӱ���̼������Һ���������Һ����ˮ�� |
| D��������Һ�����ƣ��ڽྻ���Թ��м�2% AgNO3��Һ1��2 mL����μ���2%ϡ��ˮ���ߵα���������ǡ���ܽ�ʱΪֹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
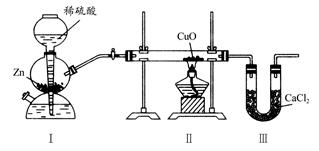
| A����װ��֮��ȱ�ٸ���װ�� |
| B����װ�ú�ȱ�ٸ���װ�� |
| C����װ���в���������ˮ���� |
| D��CuOû��ȫ������ԭ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
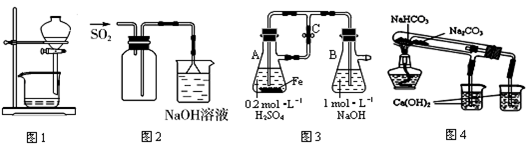
| A��ͼ1���ڷų�������Ȼ�̼��Һ |
| B��ͼ2����ʵ�����ռ�SO2 |
| C��ͼ3����ʵ�����Ʊ�Fe(OH)2 |
| D��ͼ4���ڱȽ�NaHCO3��Na2CO3���ȶ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
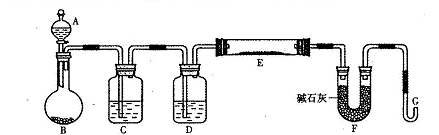
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
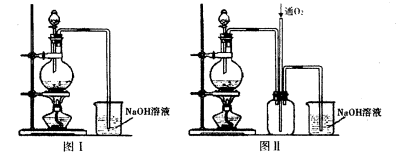
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ���������� | ���� |
| A | ������¯ˮ���е�CaSO4ʱ�����μ��뱥��Na2CO3��Һ�����ᣬˮ���ܽ� | Ksp��CaCO3��CaSO4 |
| B | ��ʯī���缫���MgSO4��Һ��ij�缫�����а�ɫ�������� | �õ缫Ϊ���� |
| C | ��FeCl3��CuCl2�����Һ�м������ۣ��к�ɫ�������� | �����ԣ�Cu2+��Fe3+ |
| D | ��ij��Һ���ȵμ������ữ���ٵμ�BaCl2��Һ���а�ɫ�������� | ����Һ��һ������Ag+ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com