
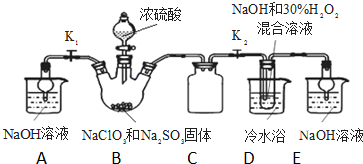
 �������ƣ�NaClO2������ҪƯ����̽��С�鿪չ����ʵ�飬�ش��������⣺
�������ƣ�NaClO2������ҪƯ����̽��С�鿪չ����ʵ�飬�ش��������⣺���� ��1����������һ��������������Һ����ѡ��������
��2��װ��C�������ǰ�ȫƿ���з��������ã�
��3��װ��B���Ʊ��õ�ClO2������B�з�ӦΪNaClO3��Na2SO3��ŨH2SO4���������� ClO2��Na2SO4���������Ⱥ��������Ʒ�Ӧ����NaClO2��
��4������Ŀ��Ϣ��֪��Ӧ�����¶�38�桫60�棬����60��ʱNaClO2�ֽ��NaClO3��NaCl��
��5����װ��D����Һ���NaClO2���壬��Ҫ�����ᾧ�����ȹ��ˡ�ϴ�ӡ����
��6��B�п��ܷ���Na2SO3+H2SO4��Ũ��=Na2SO4+SO2��+H2O��������SO2 ������D�У�SO2��H2O2 ��Ӧ���������ƣ�
��7���ٵ������۱���ɫ����Ӧ����ʱ���ⷴӦ��ȫ���μ����һ��Na2S2O3��Һʱ��Һ��ɫǡ����ȥ�Ұ�����ڲ���ԭ��˵������ζ��յ㣻
�ڸ��ݻ�ѧ��Ӧ�ɵù�ϵʽ��NaClO2��2I2��4S2O32-������Ʒ��NaClO2�����ʵ���x�����ݹ�ϵʽ���㣮
��� �⣺��1����50%˫��ˮ����30%��H2O2��Һ����Ҫ����������Ͳ���ձ���������������ιܣ����Ի���Ҫ��Ͳ��
�ʴ�Ϊ����Ͳ��
��2��װ��C�������ǰ�ȫƿ����ֹDƿ��Һ������Bƿ�У�
�ʴ�Ϊ����ֹDƿ��Һ������Bƿ�У���ȫƿ����
��3��װ��B���Ʊ��õ�ClO2������B�з�ӦΪNaClO3��Na2SO3��ŨH2SO4���������� ClO2��Na2SO4����Ӧ�ķ���ʽΪ2NaClO3+Na2SO3+H2SO4=2 ClO2��+2Na2SO4+H2O���������Ⱥ�˫��ˮ���������Ʒ�Ӧ����NaClO2����Ӧ����ʽΪ��2ClO2+2NaOH+H2O2=2NaClO2+O2+2H2O��
�ʴ�Ϊ��2ClO2+2NaOH+H2O2=2NaClO2+O2+2H2O��
��4������Ŀ��Ϣ��֪��Ӧ�����¶�38�桫60�棬����60��ʱNaClO2�ֽ��NaClO3��NaCl�����������ȥD�е���ˮԡ�����ܵ��²�Ʒ�л��е�������NaClO3��NaCl��
�ʴ�Ϊ��NaClO3��NaCl��
��5����װ��D����Һ���NaClO2���壬��Ҫ�����ᾧ�����ȹ��ˡ�ϴ�ӡ�������������Ե�iii��������45�����ҵ���ˮϴ��3�飨��ˮ�¶ȸ���38�棬����60�棩���ʴ�Ϊ����45�����ҵ���ˮϴ��3�飨��ˮ�¶ȸ���38�棬����60�棩��
��6��B�п��ܷ���Na2SO3+H2SO4��Ũ��=Na2SO4+SO2��+H2O��������SO2 ������D�У�SO2��H2O2 ��Ӧ���������ƣ�Ũ�����ѻӷ������������ѻӷ����Σ��������D����a��ȷ��b��c����ѡ��a��
��7���ٵ������۱���ɫ����Ӧ����ʱ���ⷴӦ��ȫ���μ����һ��Na2S2O3��Һʱ��Һ��ɫǡ����ȥ�Ұ�����ڲ���ԭ��˵������ζ��յ㣬
�ʴ�Ϊ���μ����һ��Na2S2O3��Һʱ����Һ��ɫǡ����ȥ�Ұ�����ڲ���ԭ��˵������ζ��յ㣻
������Ʒ��NaClO2�����ʵ���x����
NaClO2��2I2��4S2O32-��
1mol 4mol
0.25x c mol•L-1��V��10-3L
��x=c•V•10-3mol��
�ʴ�Ϊ��c•V•10-3��
���� ���⿼�����������Ʊ�ʵ��Ļ����������������Ƶ����ʼ��к͵ζ���֪ʶ����Ŀ�Ѷ��еȣ�����ԭ���ǽ���Ĺؼ���ͬʱ����ѧ���������⡢���������������ѵ�������ͼ�ķ�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������������ˮ������ | B�� | þ�����������źŵ������ | ||
| C�� | �����������������ھ�ˮ | D�� | ������������Ư��ֽ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
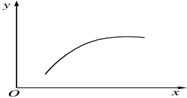 ��һ�ܱ�������ͨ��A��B�������壬��һ�������·�����Ӧ2A��g��+B��g��?2C��g����H��0������Ӧ�ﵽƽ��ı�һ��������x�����±�������y��һ������ͼ�����ߵ��ǣ�������
��һ�ܱ�������ͨ��A��B�������壬��һ�������·�����Ӧ2A��g��+B��g��?2C��g����H��0������Ӧ�ﵽƽ��ı�һ��������x�����±�������y��һ������ͼ�����ߵ��ǣ�������| ѡ�� | x | y |
| A | �¶� | ��������ƽ����Է������� |
| B | ѹǿ | A�İٷֺ��� |
| C | ��ͨ��A | B��ת���� |
| D | ������� | A���������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | $\frac{c��O{H}^{-}��}{c��C{H}_{3}CO{O}^{-}��}$ | B�� | $\frac{c��C{H}_{3}CO{O}^{-}��}{c��{H}^{+}��}$ | ||
| C�� | $\frac{c��C{H}_{3}COOH��}{c��C{H}_{3}CO{O}^{-}��}$ | D�� | c��H+��•c��CH3COO-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | HBr��KOH��CaCO3��H2O����ǿ����� | |
| B�� | Na2O2��CaO��Al2O3���Ǽ��������SiO2��SO2��NO2�������������� | |
| C�� | 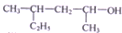 ������Ϊ4-��-2-���� ������Ϊ4-��-2-���� | |
| D�� | ${\;}_{94}^{238}$Pu��������Ϊ94��������Ϊ238��������Ϊ144 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2gD2O��2gH218O���е���������ΪNA | |
| B�� | 1L0.1mol•L-1Na2S��Һ��S2-��H2S����Ŀ֮��Ϊ0.1NA | |
| C�� | Ư���м���Ũ���ᣬÿ����1molCl2��ת�Ƶĵ�����2NA | |
| D�� | �����£���1molNO2�����ѹ����N2O4�ķ�����Ϊ0.5NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | ���� |
| ��ʢ��4g CaO2�Ĵ��Թ��м���10mLϡ�������Һa | ���ҷ�Ӧ��������ʹ������ľ����ȼ������ |
| ȡ5mL��Һa���Թ��У���������ʯ�� | ��Һ��죬һ��ʱ�����Һ��ɫ���Ա�dz���Ժ���Һ��Ϊ��ɫ |
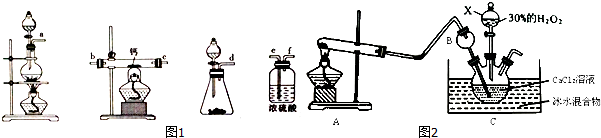
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Һ��ֻ����2�ֻ�����ķ��� | |
| B�� | ͨ��CO2ʱ��Һ��Ư�����ü��� | |
| C�� | ����Һ��A13+��Fe2+��SO42-��NO3-���Դ������� | |
| D�� | ��SO2��Ӧ�����ӷ���ʽΪCa2++2ClO-+SO2+H2O=CaSO3��+2HClO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
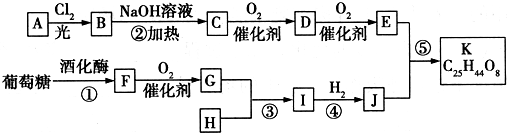
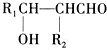
 ��G��CH3CHO��
��G��CH3CHO��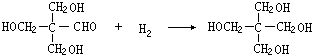 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com