
��08�������Ǽ�⣩���������Ȼ�ѧ����ʽ�ó��Ľ�����ȷ���ǣ� ��
A.��֪2H2(g) + O2(g) == 2H2O(g); ��H=-483.6kJ?mol��1 ����2g����ȼ������Һ̬ˮʱ����Ϊ241.8 kJ?mol��1
B.��֪C��ʯī��s��=C�����ʯ��s��,��H>0 ����ʯ��ʯī�ȶ�
C.��֪2C(s) + 2O2(g) == 2CO2(g)����H1 2C(s) + O2(g) == 2CO(g)����H2�����H1>��H2
D.��֪NaOH(aq) + HCl(aq) == NaCl(aq) + H2O(l); ��H=-57.6 kJ?mol��1����20.0gNaOH��ϡ��Һ��ϡ������ȫ�кͣ��ų�28.7kJ������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08�������Ǽ�⣩ʵ�������ĸ�ҩƷ�����Ѵ������ҩƷ
ҩƷ�� | �׳� | �ҳ� | ���� | ���� |
ҩƷ | ���ᡢ���� | �������ơ��������� | ���ס��� | ͭ��п |
ʵ�����¹���һЩ����̿��Ӧ��������ڣ� ��
A.�׳� B.�ҳ� C.���� D.����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08�������Ǽ�⣩������ԭ��Ӧ�������������о��й㷺����;�����������������е�����������������ԭ��Ӧ���ǣ� ��
A.����ұ�� B.���ƶ��� C.ʳ�︯�� D.ȼ�ű���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08�������Ǽ�⣩.����ͼװ��ʵ�飬��x���ʾ���������ĵ��ӵ����ʵ�������y��ɱ�ʾ�� ��
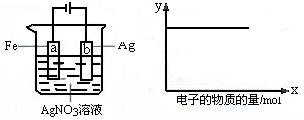
��c(Ag+) ��c(AgNO3) ��a�������� ��b�������� ����Һ��pH
A.�٢� B.�ۢ� C.�٢ڢ� D.�٢ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08�������Ǽ�⣩���и�������һ���ܴ���������ǣ� ��
A.��ǿ������Һ�У�Na+��K+��[Al(OH)4]����CO32��
B.�ں��д���Fe3+����Һ�У�NH4+��Na+��Cl����SCN��
C.��c(H+)=10��13mol?l��1����Һ�У�NH4+��Al3+��SO42����NO3��
D.��pH=1����Һ�У�K+��Fe2+��Cl����NO3��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com