
·ÖĪö £Ø1£©ÓĆpHŹŌÖ½¼ģŃ飬±ķĆ÷ČÜŅŗ³ŹĻÖĒæĖįŠŌ£¬Ņ»¶Øŗ¬ÓŠH+£¬CO32-Äܹ»ÓėĒāĄė×Ó·“Ó¦£¬ŌŚČÜŅŗÖŠ²»»į“ęŌŚ£»
£Ø2£©ĖÄĀČ»ÆĢ¼ČÜŅŗ³Ź×ĻŗģÉ«£¬ĖµĆ÷¼ÓČėĀČĖ®ŗóÓŠµāµ„ÖŹÉś³É£¬ŌČÜŅŗÖŠŅ»¶Øŗ¬ÓŠI-£»
£Ø3£©¼ÓČėĒāŃõ»ÆÄĘČÜŅŗµÄ¹ż³Ģ֊ƻӊ³ĮµķÉś³É£¬ĖµĆ÷Ņ»¶Ø²»“ęŌŚMg2+£»
£Ø4£©Ba2+ÄÜÓėĢ¼ĖįÄĘ·“Ó¦²śÉś³ĮµķĄ“ÅŠ¶Ļ“ęŌŚµÄĄė×Ó£¬Ņ»¶Ø“ęŌŚBa2+£¬øł¾ŻĄė×Ó¹²“ęÅŠ¶Ļ²»ÄÜ“ęŌŚµÄĄė×Ó£®
½ā“š ½ā£ŗ£Ø1£©ÓĆpHŹŌÖ½¼ģŃ飬±ķĆ÷ČÜŅŗ³ŹĻÖĒæĖįŠŌ£¬Ņ»¶Øŗ¬ÓŠH+£¬CO32-Äܹ»ÓėĒāĄė×Ó·“Ó¦£¬ŌŚČÜŅŗÖŠ²»»į“ęŌŚ£»
£Ø2£©ĖÄĀČ»ÆĢ¼ČÜŅŗ³Ź×ĻŗģÉ«£¬ĖµĆ÷¼ÓČėĀČĖ®ŗóÓŠµāµ„ÖŹÉś³É£¬ŌČÜŅŗÖŠŅ»¶Øŗ¬ÓŠI-£»
£Ø3£©¼ÓČėĒāŃõ»ÆÄĘČÜŅŗµÄ¹ż³Ģ֊ƻӊ³ĮµķÉś³É£¬ĖµĆ÷Ņ»¶Ø²»“ęŌŚMg2+£»
£Ø4£©Ba2+ÄÜÓėĢ¼ĖįÄĘ·“Ó¦²śÉś³ĮµķĄ“ÅŠ¶Ļ“ęŌŚµÄĄė×Ó£¬Ņ»¶Ø“ęŌŚBa2+£¬Ņ»¶Ø²»“ęŌŚCO32-”¢SO42-£¬NH4+ĪŽ·ØÅŠ¶ĻŹĒ·ń“ęŌŚ£®
¹Ź“š°øĪŖ£ŗH+”¢I-”¢Ba2+£»CO32-”¢Mg2+”¢SO42-£»NH4+£®
µćĘĄ ±¾ĢāÖ÷ŅŖæ¼²éĮĖ³£¼ūĄė×ӵļģŃé·½·Ø£¬ĢāÄæÄѶČÖŠµČ£¬×¢ŅāÕĘĪÕ³£¼ūĄė×ӵĻÆѧŠŌÖŹ¼°¼ģŃé·½·Ø£¬Äܹ»øł¾ŻĄė×Ó¹²“ę”¢Ąė×Ó·“Ó¦ĻÖĻóÅŠ¶ĻĄė×Ó¹²“ęĒéæö£¬Ć÷Č·¼ģŃéĄė×ÓŹ±£¬±ŲŠėÅųżøÉČÅĄė×Ó£¬Č·±£¼ģŃé·½°øµÄŃĻĆÜŠŌ£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Na | B£® | Fe | C£® | Cu | D£® | Al |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | A£¬B·Ö±šĪŖ0.4mol•L-1£¬0.2mol•L-1 | B£® | AĪŖ0.25mol•L-1 | ||
| C£® | A£¬C¾łĪŖ0.15mol•L-1 | D£® | AĪŖ0.24mol•L-1£¬CĪŖ0.14mol•L-1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | Ō»ģŗĻČÜŅŗÖŠÖ»“ęŌŚNa+”¢Fe3+”¢SO42-£¬²»æÉÄÜ“ęŌŚK+”¢CO32- | |
| B£® | ÓÉŹµŃé£Ø1£©ĪŽ·ØĶʶĻŌ»ģŗĻČÜŅŗÖŠŹĒ·ńŗ¬ÓŠSO42- | |
| C£® | ÓÉŹµŃé£Ø2£©ĪŽ·ØĶʶĻŌ»ģŗĻČÜŅŗÖŠŹĒ·ńŗ¬ÓŠFe3+ | |
| D£® | ÓÉŹµŃé£Ø3£©æÉĶʶĻŌ»ģŗĻČÜŅŗÖŠ“ęŌŚFe2+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
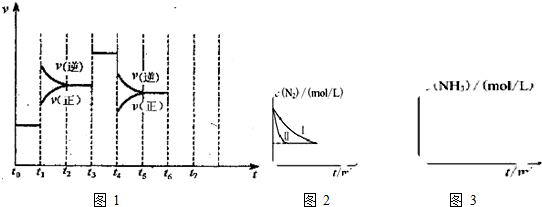
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | HCl | B£® | NaOH | C£® | Na2SO4 | D£® | NaCl |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
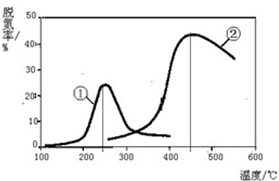 ½ųČė¶¬¼¾±±·½æŖŹ¼¹©ÅÆŗó£¬Īķö²ĢģĘųÓś·¢ŃĻÖŲ£¬ø÷µŲPM2.5”¢PM10¾³£”°±¬±ķ”±£®Ņż·¢Īķö²ĢģĘųµÄĪŪČ¾ĪļÖŠ£¬×īĪŖ³£¼ūµÄŹĒ»ś¶Æ³µĪ²ĘųÖŠµÄµŖŃõ»ÆĪļŗĶČ¼Ćŗ²śÉśµÄŃĢĘų£®
½ųČė¶¬¼¾±±·½æŖŹ¼¹©ÅÆŗó£¬Īķö²ĢģĘųÓś·¢ŃĻÖŲ£¬ø÷µŲPM2.5”¢PM10¾³£”°±¬±ķ”±£®Ņż·¢Īķö²ĢģĘųµÄĪŪČ¾ĪļÖŠ£¬×īĪŖ³£¼ūµÄŹĒ»ś¶Æ³µĪ²ĘųÖŠµÄµŖŃõ»ÆĪļŗĶČ¼Ćŗ²śÉśµÄŃĢĘų£®Ź±¼ä/min ĪĀ¶Č/”ę | 0 | 10 | 20 | 40 | 50 |
| T1 | 1.2 | 0.9 | 0.7 | 0.4 | 0.4 |
| T2 | 1.2 | 0.8 | 0.56 | ” | 0.5 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
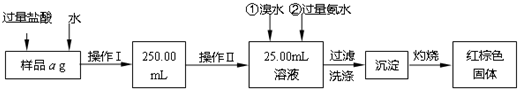
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com