
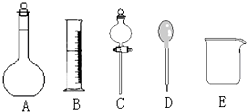
 ��У������ȤС���ڴ�����ˮ��Ʒʱ��������������Ϊ36.5%��Ũ���ᣨ�ܶ�Ϊ1.19g/cm3�����Ƴ�240mL 0.1mol•L-1��������Һ��
��У������ȤС���ڴ�����ˮ��Ʒʱ��������������Ϊ36.5%��Ũ���ᣨ�ܶ�Ϊ1.19g/cm3�����Ƴ�240mL 0.1mol•L-1��������Һ������ ��1����������һ�����ʵ���Ũ�ȵ���Һʹ�õ�����������Ҫ��������ȱ�ٵ��������ٸ��ݲ������ڲ����е����ý��
��2������c=$\frac{1000�Ѧ�}{M}$�������ҪŨ�����Ũ�ȣ��ٸ�������250mL 0.1mol•L-1��������Һ��Ҫ���Ȼ�������ʵ����������Ҫ�����������250mL��Һ��Ҫ250mL����ƿ��
��3���������Ʋ����Ǽ��㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ���������Ʋ��裻
��4����������ƿ����ȷʹ�÷��������жϣ�
��5������c=$\frac{n}{V}$��������ʵ����ʵ���n����Һ�����V�ı仯��������������
��� �⣺��1����Һ©��������ȡ�ͷ�Һ������һ�����ʵ���Ũ����Һ���÷�Һ©������ѡC����ȱ�ٲ���������Ũ����ϡ��ʱ�ò��������裬ת��Һ��ʱ�ò�����������
�ʴ�Ϊ��C�������������裻������
��2����������Ϊ37%��Ũ���ᣨ�ܶ�Ϊ1.19g/cm3�������ʵ���Ũ��Ϊ��c=$\frac{1000�Ѧ�}{M}$=$\frac{1000��1.19��37%}{36.5}$=12.1��mol/L��������Ũ�������V=$\frac{0.1mol/L��0.25L}{12.1mol/L}$=0.0021L=2.1mL����ѡ��10mL��Ͳ������250mL��Һ��Ӧѡ��250mL������ƿ��
�ʴ�Ϊ��2.1��A��C��
��3���������Ʋ����Ǽ��㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��֪��ȷ�IJ��������Ǣۢ٢ܢڣ��ʴ�Ϊ���ۢ٢ܢڣ�
��4��A������ƿʹ��ʱ��Ӧ�ȼ���Ƿ�©ˮ��Ȼ��������ˮϴ�Ӹɾ����ɣ���A��ȷ��
B������ƿϴ����������������Һ��ϴ������Ӱ�����Ƶ���Һ��Ũ�ȣ���B����
C������ƿֻ������������Һ������������ƿ���ܽ⣬Ӧ�����ձ����ܽ⣬��C����
D������ƿֻ������������Һ������������ƿ��ϡ�ͣ�Ӧ�����ձ���ϡ�ͣ���D����
E��ҡ��ʱ���Ǻ�ƿ������ʳָ��סƿ������һֻ����סƿ�ף�������ƿ������ת����E��ȷ��
��ѡAE��
��5��������ƿ������������ˮ����������Һ��Ũ����Ӱ�죬��ѡc��
���ձ��Ͳ���û��ϴ��2-3�Σ���ᵼ�����ʵ���ʧ��Ũ��ƫ�ͣ���ѡb��
��ϡ��ŨHClʱ��û����ȴ������ת�Ƶ�����ƿ�У�����ȴ����Һ���ƫС��Ũ��ƫ�ߣ���ѡa��
�����Ƶ���Һװ��ྻ�ĵ�����������ˮ���Լ�ƿ�У������Һ���ϡ�ͣ���Ũ��ƫ�ͣ���ѡb��
��������ʱ���ӣ�����Һ���ƫС��������Һ�����ʵ���Ũ��ƫ�ߣ���ѡa��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���Ŀ�ѶȲ���Ҫ��ѧ��������������һ�����ʵ���Ũ�ȵ���Һ�ķ�������ɷ���������ע��������ɸ���c=$\frac{n}{V}$���з����������ʵ����ʵ���n�������ƽ��ƫ�ߣ�����Һ�����V���������Ƶ���ҺŨ��ƫ�ͣ�


 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 5.6������Ӧת�Ƶĵ�����һ��Ϊ3NA | |
| B�� | 1mol Cl2��Ӧת�Ƶĵ�����һ����2NA | |
| C�� | ��״���£�22.4L SO3���е���ԭ����ĿΪ3NA | |
| D�� | 1mol̼������CH5+�����ĵ�������Ϊ10NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ˮ�� | B�� | ǿ���� | C�� | ��ˮ�� | D�� | ǿ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ʵ���ҿ����ö������̺�Ũ�����ڼ����������Ʊ���������ͼΪ
ʵ���ҿ����ö������̺�Ũ�����ڼ����������Ʊ���������ͼΪ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | $\frac{a+b}{4}$ | B�� | $\frac{3a+2b}{4}$ | C�� | 4��a+b�� | D�� | 4��3a+b�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ƽ�ⳣ����С | B�� | ����A��ת���������� | ||
| C�� | ƽ��������Ӧ�����ƶ��� | D�� | a��b |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ֱͨ���罺���������ƶ����ǵ������Һֱͨ�������ʲ��ƶ� | |
| B�� | ��Һ������������ͨ����ֽ�������з�ɢ�����Ӳ���ͨ����ֽ | |
| C�� | ��Һ��ͨ��һ������û��������������ͨ��һ�����߳������Թ�� | |
| D�� | ��Һ�ȶ������ú����ɳ��������岻�ȶ������û����ɳ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڲⶨ��Һ��pHʱ����������ˮʪ���ò�������ȡ��Һ������ֽ�в����������ɫ���Ƚ� | |
| B�� | ij��Һ��BaCl2��Һ���ɰ�ɫ������˵��ԭ��Һ����SO42- | |
| C�� | �ᴿ��������Ba��NO3��2���ʵ�KNO3��Һ������ʹ�õķ���Ϊ���������K2CO3��Һ�����˳�ȥ������������Һ�в�������HNO3 | |
| D�� | ʵ��������ò�����ƺͰ���Ӧֱ���ӵ���Һ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
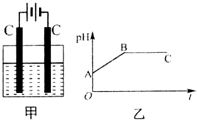 ��װ���������������ʵ���֮��Ϊ1��2��CuSO4��NaCl�Ļ����Һ������������Һ��pHֵ��ʱ��t�仯��ʾ��ͼ����ʾ�������ǵ����������ˮ�ķ�Ӧ�����Է���������������ȷ���ǣ�������
��װ���������������ʵ���֮��Ϊ1��2��CuSO4��NaCl�Ļ����Һ������������Һ��pHֵ��ʱ��t�仯��ʾ��ͼ����ʾ�������ǵ����������ˮ�ķ�Ӧ�����Է���������������ȷ���ǣ�������| A�� | �Ǹû����Һ�е�SO42-������A����Һ��pHֵС��B�� | |
| B�� | BC�����������������������֮��Ϊ2��1 | |
| C�� | AB�߶���BC�߶��������Ϸ����ķ�Ӧ����ͬ�ļ���Cu2++2e-��Cu | |
| D�� | ���������Ĺ����л������������ɫ��Cu��OH��2���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com