
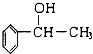
 ����Ϊͬϵ�
����Ϊͬϵ�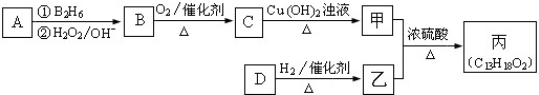
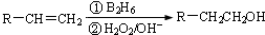
 �����Ϊ
�����Ϊ ��D�ɷ���������Ӧ���ڴ���������1mol D��2mol H2���Է�Ӧ�����ң�����DΪC9H8O����D�Ľṹ��ʽΪ
��D�ɷ���������Ӧ���ڴ���������1mol D��2mol H2���Է�Ӧ�����ң�����DΪC9H8O����D�Ľṹ��ʽΪ ���ݴ˽��
���ݴ˽�� �����Ϊ
�����Ϊ ��D�ɷ���������Ӧ���ڴ���������1mol D��2mol H2���Է�Ӧ�����ң�����DΪC9H8O����D�Ľṹ��ʽΪ
��D�ɷ���������Ӧ���ڴ���������1mol D��2mol H2���Է�Ӧ�����ң�����DΪC9H8O����D�Ľṹ��ʽΪ ��
��| �� |
| �� |
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
| Ũ����� |
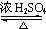
 +H2O��
+H2O��
| Ũ���� |
| �� |
 +H2O��
+H2O�� ����ͬ���칹����ϣ���������3��ȡ����������ţ��������ԣ������Ϊ-OH��-CH3��-CH2CH3�������� 3��ȡ����������Ż������ڣ����ͬ���칹��Ϊ
����ͬ���칹����ϣ���������3��ȡ����������ţ��������ԣ������Ϊ-OH��-CH3��-CH2CH3�������� 3��ȡ����������Ż������ڣ����ͬ���칹��Ϊ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��


 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ƫ�����µ�Ħ������Ϊ60 g?mol-1 |
| B��6.02��1023��ƫ�����·��ӵ�����Ϊ60 |
| C��1 molƫ�����µ�����Ϊ60 g?mol-1 |
| D��6 gƫ�����º���NA��ƫ�����·��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������ϡ���ᷴӦ��2Fe+6H+=2Fe3++3H2�� |
| B�������е���̼������Һ��CO32-+2H+=H2O+CO2�� |
| C�������е�������������Һ��HCl+OH-=H2O+Cl- |
| D��ϡ�������ʯ��ʯ�ϣ�CO32-+2H+=H2O+CO2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��� | ʵ����� | ʵ������ | ��������� |
| A | ��ij��Һ�м���AgNO3��Һ | �а�ɫ�������� | ������������ˮ��AgCl�� ����Һ��һ������Cl- |
| B | ������Һ�У� ���뱥�ͣ�NH4��2SO4��Һ | �а�ɫ�������� | �����ʷ��������� |
| C | ��ױ��е�������Ũ��ˮ�������� | ��Һ�ֲ㣬�ϲ�ʳȺ�ɫ���²㼸����ɫ | �ױ�����ˮ����ȡ����Ӧ�� ʹ��ˮ��ɫ |
| D | �������м���Ũ���� | ��ڣ����ȣ�������ͣ��ų��̼������� | Ũ���������ˮ�Ժ�ǿ�����ԣ� ��Ӧ������C��SO2��CO2�� |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
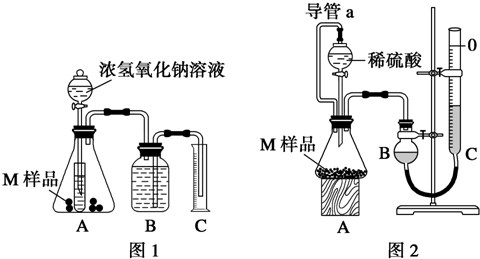
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��H+��Ca2+��Fe3+��NO3- |
| B��Ba2+��Cl-��Al3+��H+ |
| C��Na+��NH4+��I-��HS- |
| D��Na+��NH3?H2O��K+��Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��A���A1O2- |
| B��A��c��C1-����c��K+�� |
| C��A��pH��7 |
| D��������NH4Cl��KOH��AgNO3 ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com