
 ������2�Ļ�ѧʽΪAl��OH��3��
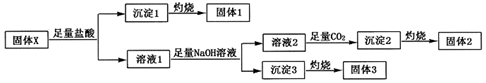
������2�Ļ�ѧʽΪAl��OH��3������ ����1������2������3�����������²��ϣ���ѧ�г��������²���Ϊ�������衢����þ���������ȣ�����X�����ᷴӦ���ɳ�������ȼ�յù���1�������1ΪSiO2������1ΪH2SiO3������X�����ᷴӦ������Һ1����Һ1�к���þ�������ӣ������������������Ƶ���Һ2�ͳ���3����Һ2����ͨ������̼�ó���2���յù���2������3���յù���3�������2ΪAl2O3������2ΪAl��OH��3������3ΪMgO������3ΪMg��OH��2��39.3g������X�õ�15.3g����2��0.15molAl2O3��6.0g����3��0.15molMgO�����廯����X�����ֳ����Ķ�����Ԫ����ɣ�����Ԫ���غ��֪��X�к���þ�������衢��Ԫ�أ���X�к��й�������������Ϊ39.3g-15.3g-6.0g=18g����X�к���SiO2�����ʵ���Ϊ0.3mol������X�����ΪMgO•Al2O3•2SiO2���ݴ˴��⣮
��� �⣺����1������2������3�����������²��ϣ���ѧ�г��������²���Ϊ�������衢����þ���������ȣ�����X�����ᷴӦ���ɳ�������ȼ�յù���1�������1ΪSiO2������1ΪH2SiO3������X�����ᷴӦ������Һ1����Һ1�к���þ�������ӣ������������������Ƶ���Һ2�ͳ���3����Һ2����ͨ������̼�ó���2���յù���2������3���յù���3�������2ΪAl2O3������2ΪAl��OH��3������3ΪMgO������3ΪMg��OH��2��39.3g������X�õ�15.3g����2��0.15molAl2O3��6.0g����3��0.15molMgO�����廯����X�����ֳ����Ķ�����Ԫ����ɣ�����Ԫ���غ��֪��X�к���þ�������衢��Ԫ�أ���X�к��й�������������Ϊ39.3g-15.3g-6.0g=18g����X�к���SiO2�����ʵ���Ϊ0.3mol������X�����ΪMgO•Al2O3•2SiO2��
��1��NaOHΪ���ӻ�������к������Ӽ����ۼ��������ʽΪ ������2�Ļ�ѧʽΪAl��OH��3��
������2�Ļ�ѧʽΪAl��OH��3��
�ʴ�Ϊ�� ��Al��OH��3��
��Al��OH��3��
��2����������ķ�����֪������X�Ļ�ѧʽΪMgO•Al2O3•2SiO2��
�ʴ�Ϊ��MgO•Al2O3•2SiO2��
��3����Һ1�м�������NaOH��Һ������������þ��ƫ��������ӣ���Ӧ�����ӷ���ʽΪ Al3++4OH-�TAlO2-+2H2O��Mg2++2OH-=Mg��OH��2����
�ʴ�Ϊ��Al3++4OH-�TAlO2-+2H2O��Mg2++2OH-=Mg��OH��2����
��4������3ΪMgO������1ΪSiO2���ڸ����£�����3��ijԪ�صĵ��ʿ��������1�����û���Ӧ���˷�Ӧ�Ļ�ѧ����ʽΪ2Mg+SiO2$\frac{\underline{\;����\;}}{\;}$2MgO+Si��
�ʴ�Ϊ��2Mg+SiO2$\frac{\underline{\;����\;}}{\;}$2MgO+Si��
���� ���⿼��Ԫ�ػ���������Ժͷ�Ӧ����Ҫ�����˹衢����þ��Ԫ�ؼ��仯�����֪ʶ������ʱע�������ѧ�������ʵ���;���ܸ������ʵ���;�жϻ������ǽⱾ��Ĺؼ�����Ŀ�Ѷ��еȣ�


 �Ƹ�С״Ԫ�������������ϵ�д�
�Ƹ�С״Ԫ�������������ϵ�д� ����һ������ܼƻ�ϵ�д�
����һ������ܼƻ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��0.1mol•L-1��Na2A��Һ�У�c��A2-��+c��HA-��+2c��Na+��=0.5mol•L-1 | |
| B�� | ��0.1mol•L-1��H2A��Һ�У�c��H+����0.12mol•L-1 | |
| C�� | ��ͬŨ�ȣ�0.1mol•L-1����NaHA��Na2A��Һ�������ϣ������Һ���ܳʼ��� | |
| D�� | 0.1mol•L-1��NaHA��Һ������Ũ��Ϊc��Na+����c��H+����c��A2-����c��OH-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ԭ�ӵĽṹʾ��ͼ��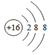 | B�� | ������Ľṹʽ��H-O-Cl | ||
| C�� | ������Ϊ7 ��̼ԭ�ӣ�${\;}_{6}^{7}$C | D�� | CO2 �ĵ���ʽ��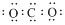 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
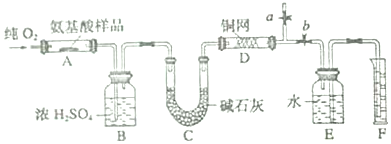
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
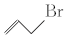 ����������
��������л���  ��˵����ȷ���У�������
��˵����ȷ���У�������| A�� | �뱽��Ϊͬ���칹�� | B�� | ����ȴ�����3�� �������������칹�� | ||
| C�� | ���������е�̼ԭ����ͬһƽ���� | D�� | ����ϩ��������ϩ��ͬϵ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | a+m+n | B�� | a+m-n | C�� | a-m+n | D�� | a-m-n |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ե���Ϊԭ�Ͽ�����ȡ�������� | |
| B�� | ��֬��������Ӧ���ڼӳɷ�Ӧ | |
| C�� | ����������ԭ�ӿ��ܴ���ͬһƽ�� | |
| D�� | ����ʽΪC3H6Cl2��ͬ���칹����5�֣������������칹�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �ڢۢ� | C�� | �ڢܢ� | D�� | �٢ڢۢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com